5.1.8. 1-methyl-3-octylimidazolium hexafluorophosphate,
[MOIM][PF6]
Table 5-26: Activity coefficients at infinite
dilution of organic solutes in 1-methyl-3-
octylimidazolium hexafluorophosphate with n3 = 6.69
mmol (28.55 %)
at T = (313.15, 323.15 and 333.15) K.
Experimental at /K
Solute
|
n3/mmol
|
T=313.15
|
T=323.15
|
T=333.15
|
n-pentane
|
6.69
|
8.21
|
8.06
|
7.83
|
n-hexane
|
6.69
|
10.75
|
10.56
|
10.20
|
n-heptane
|
6.69
|
14.07
|
13.78
|
13.24
|
n-octane
|
6.69
|
18.34
|
17.87
|
17.04
|
n-decane
|
6.69
|
31.75
|
30.25
|
28.23
|
n-undecane
|
6.69
|
42.06
|
39.62
|
36.52
|
Hex-1-ene
|
6.69
|
6.17
|
6.15
|
6.06
|
Hept-1-ene
|
6.69
|
8.23
|
8.18
|
8.02
|
Oct-1-ene
|
6.69
|
10.77
|
10.66
|
10.39
|
Non-1-ene
|
6.69
|
14.16
|
13.93
|
13.50
|
Dec-1-ene
|
6.69
|
16.32
|
16.52
|
16.34
|
Undec-1-ene
|
6.69
|
25.10
|
24.01
|
22.75
|
Pent-1-yne
|
6.69
|
1.70
|
1.75
|
1.78
|
Hex-1-yne
|
6.69
|
2.24
|
2.30
|
2.34
|
Hept-1-yne
|
6.69
|
2.81
|
2.87
|
2.93
|
Oct-1-yne
|
6.69
|
3.77
|
3.87
|
3.94
|
Non-1-yne
|
6.69
|
4.38
|
4.65
|
4.83
|
Cyclopentane
|
6.69
|
5.02
|
4.92
|
4.77
|
Cyclohexane
|
6.69
|
6.74
|
6.56
|
6.29
|
Cycloheptane
|
6.69
|
8.24
|
7.97
|
7.63
|
Cyclooctane
|
6.69
|
10.21
|
9.85
|
9.39
|
Methanol
|
6.69
|
1.77
|
1.61
|
1.46
|
Ethanol
|
6.69
|
2.22
|
2.00
|
1.79
|
Benzene
|
6.69
|
0.96
|
0.99
|
1.00
|
Toluene
|
6.69
|
1.31
|
1.35
|
1.37
|
Ethylbenzene
|
6.69
|
1.95
|
1.98
|
1.99
|
|
Table 5-27: Activity coefficients at infinite
dilution of organic solutes in 1-methyl-3-
octylimidazolium hexafluorophosphate with n3 = 5.135
mmol (33.26 %)
at T = (313.15, 323.15 and 333.15) K.
Experimental at /K
Solute
|
n3/mmol
|
T=313.15
|
T=323.15
|
T=333.15
|
n-pentane
|
5.14
|
8.32
|
8.11
|
7.99
|
n-hexane
|
5.14
|
11.01
|
10.68
|
10.41
|
n-heptane
|
5.14
|
14.30
|
13.59
|
13.21
|
n-octane
|
5.14
|
17.64
|
17.09
|
16.93
|
n-decane
|
5.14
|
31.68
|
30.80
|
28.98
|
n-undecane
|
5.14
|
41.33
|
40.13
|
36.99
|
Hex-1-ene
|
5.14
|
6.45
|
6.29
|
6.15
|
Hept-1-ene
|
5.14
|
8.19
|
8.12
|
8.14
|
Oct-1-ene
|
5.14
|
10.30
|
10.23
|
10.34
|
Non-1-ene
|
5.14
|
13.48
|
13.25
|
13.28
|
Dec-1-ene
|
5.14
|
16.66
|
16.30
|
16.36
|
Undec-1-ene
|
5.14
|
25.24
|
24.79
|
22.52
|
Pent-1-yne
|
5.14
|
1.72
|
1.79
|
1.84
|
Hex-1-yne
|
5.14
|
2.33
|
2.38
|
2.43
|
Hept-1-yne
|
5.14
|
2.93
|
2.98
|
3.04
|
Oct-1-yne
|
5.14
|
3.90
|
3.94
|
4.03
|
Non-1-yne
|
5.14
|
4.47
|
4.71
|
4.91
|
Cyclopentane
|
5.14
|
5.06
|
4.95
|
4.79
|
Cyclohexane
|
5.14
|
6.82
|
6.61
|
6.30
|
Cycloheptane
|
5.14
|
8.02
|
7.74
|
7.75
|
Cyclooctane
|
5.14
|
9.92
|
9.51
|
9.37
|
Methanol
|
5.14
|
1.85
|
1.65
|
1.50
|
Ethanol
|
5.14
|
2.32
|
2.05
|
1.82
|
Benzene
|
5.14
|
0.97
|
0.99
|
1.03
|
Toluene
|
5.14
|
1.36
|
1.37
|
1.41
|
Ethylbenzene
|
5.14
|
1.95
|
1.98
|
2.02
|
|
Table 5-28: Average activity coefficients at
infinite dilution of organic solutes in 1-
methyl-3-octylimidazolium hexafluorophosphate at T =
(313.15, 323.15 and 333.15) K.
Experimental
|
at /K
|
|
Solute
|
T=313.15
|
T=323.15
|
T=333.15
|
n-pentane
|
8.27
|
8.09
|
7.91
|
n-hexane
|
10.88
|
10.62
|
10.31
|
n-heptane
|
14.19
|
13.69
|
13.23
|
n-octane
|
17.99
|
17.48
|
16.99
|
n-decane
|
31.72
|
30.53
|
28.61
|
n-undecane
|
41.70
|
39.88
|
36.76
|
Hex-1-ene
|
6.31
|
6.22
|
6.11
|
Hept-1-ene
|
8.21
|
8.15
|
8.08
|
Oct-1-ene
|
10.54
|
10.45
|
10.37
|
Non-1-ene
|
13.82
|
13.59
|
13.39
|
Dec-1-ene
|
16.49
|
16.41
|
16.35
|
Undec-1-ene
|
25.17
|
24.40
|
22.64
|
Pent-1-yne
|
1.71
|
1.77
|
1.81
|
Hex-1-yne
|
2.29
|
2.34
|
2.39
|
Hept-1-yne
|
2.87
|
2.93
|
2.99
|
Oct-1-yne
|
3.84
|
3.91
|
3.99
|
Non-1-yne
|
4.43
|
4.68
|
4.87
|
Cyclopentane
|
5.04
|
4.94
|
4.78
|
Cyclohexane
|
6.78
|
6.59
|
6.30
|
Cycloheptane
|
8.13
|
7.86
|
7.69
|
Cyclooctane
|
10.07
|
9.68
|
9.38
|
Methanol
|
1.81
|
1.63
|
1.48
|
Ethanol
|
2.27
|
2.03
|
1.81
|
Benzene
|
0.97
|
0.99
|
1.02
|
Toluene
|
1.34
|
1.36
|
1.39
|
Ethylbenzene
|
1.95
|
1.98
|
2.01
|
|
Table 5-29: Excess molar enthalpies at
infinite dilution of organic solutes for the ionic
liquid 1-methyl-3-octylimidazolium hexafluorophosphate,
calculated using the Gibbs-
Helmholtz equation.
SOLUTE
|
Linear regression using Eq.(2-11)
|
|
|
|
n-pentane
|
0.229
|
1.381
|
0.999
|
1.90
|
n-hexane
|
0.283
|
1.485
|
0.994
|
2.35
|
n-heptane
|
0.366
|
1.485
|
1.000
|
3.04
|
n-octane
|
0.300
|
1.933
|
0.999
|
2.49
|
n-decane
|
0.537
|
1.748
|
0.973
|
4.46
|
n-undecane
|
0.656
|
1.643
|
0.966
|
5.45
|
Hex-1-ene
|
0.172
|
1.294
|
0.991
|
1.43
|
Hept-1-ene
|
0.083
|
1.840
|
0.996
|
0.69
|
Oct-1-ene
|
0.085
|
2.084
|
0.999
|
0.71
|
Non-1-ene
|
0.165
|
2.099
|
0.999
|
1.37
|
Dec-1-ene
|
0.045
|
2.660
|
0.996
|
0.37
|
Undec-1-ene
|
0.551
|
1.473
|
0.937
|
4.58
|
Pent-1-yne
|
-0.297
|
1.487
|
0.989
|
-2.47
|
Hex-1-yne
|
-0.224
|
1.541
|
0.998
|
-1.86
|
Hept-1-yne
|
-0.205
|
1.708
|
0.999
|
-1.70
|
Oct-1-yne
|
-0.200
|
1.982
|
0.997
|
-1.66
|
Non-1-yne
|
-0.501
|
3.088
|
0.994
|
-4.16
|
Cyclopentane
|
0.276
|
0.739
|
0.982
|
2.29
|
Cyclohexane
|
0.386
|
0.684
|
0.980
|
3.21
|
Cycloheptane
|
0.291
|
1.165
|
0.986
|
2.42
|
Cyclooctane
|
0.368
|
1.133
|
0.998
|
3.06
|
Methanol
|
1.050
|
-2.760
|
1.000
|
8.73
|
Ethanol
|
1.195
|
-2.996
|
0.999
|
9.94
|
Benzene
|
-0.263
|
0.806
|
0.999
|
-2.19
|
Toluene
|
-0.210
|
0.960
|
0.996
|
-1.75
|
Ethylbenzene
|
-0.145
|
1.132
|
0.999
|
-1.21
|
|
2.95 3 3.05 3.1 3.15 3.2 3.25
1000K/T

4
3.5
111( EP13)
3
2.5
2
1.5
Figure 5-48: Plots of versus for n-alkanes in
[MOIM] [PF6] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
n-pentane, () n-hexane,
(?) n-heptane, (?) n-octane, () n-decane and (?)
n-undecane.
2.95 3 3.05 3.1 3.15 3.2 3.25
1000/T/K-1
Figure 5-49: Plots of versus for alk-1-enes in
[MOIM] [PF6] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
hex-1-ene, () hept-1-ene,
(?) oct-1-ene, (?) non-1-ene, () dec-1-ene and (?)
undec-1-ene.

2.95 3 3.05 3.1 3.15 3.2 3.25
1000K/T
In(L13)
0.8
0.6
0.4
1.8
1.6
1.4
1.2
1
Figure 5-50: Plots of versus for alk-1-ynes in
[MOIM] [PF6] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
pent-1-yne, () hex-1-yne,
(?) hept-1-yne, (?) oct-1-yne and () non-1-yne.
2.95 3 3.05 3.1 3.15 3.2 3.25
1000K/T
2.5
2
ln( L13)
1.5
1
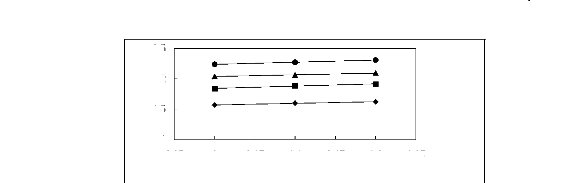
Figure 5-51: Plots of versus for cycloalkanes in
[MOIM] [PF6] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
cyclopentane, () cyclohexane,
(?) cycloheptane and (?) cyclooctane.
2.95 3 3.05 3.1 3.15 3.2 3.25
1000K/T
In( EF13)
0.8
0.6
0.4
0.2
1
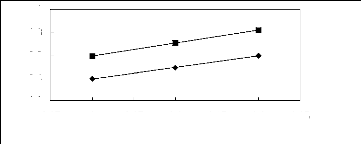
Figure 5-52: Plots of versus for alkanols in
[MOIM] [PF6] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
methanol and () ethanol.
-0.22.95 3 3.05 3.1 3.15 3.2 3.25
1000K/T
In( E1:13)
0.8
0.6
0.4
0.2
0
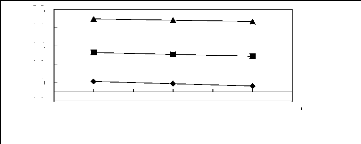
Figure 5-53: Plots of versus for alkylbenzenes
in [MOIM] [PF6] together with a linear
correlation of the data using the Gibbs-Helmholtz equation; ()
benzene, () toluene and
(?) ethylbenzene.
0 1 2 3 4 5 6 7 8 9 10 11 12 13
4
3
In( EF13)
2
1
0
-1
Nc
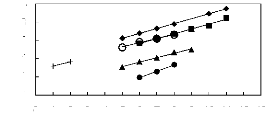
Figure 5-54: Plots of versus the number of
carbon atoms at 313.15 K for () n-alkanes,
() alk-1-enes, (?) alk-1-ynes, and (?) cycloalkanes, (+)
alkanols and (?) alkylbenzenes in
[MOIM] [PF6]
5.2. Results from the inert gas stripping
technique
5.2.1. N-methyl-2-pyrrolidone, NMP
Table 5-30: Experimental infinite dilution
activity coefficients of n-hexane as well as cyclohexane in NMP obtained by the
dilutor method and comparison with literature data taken from Gruber et al.
(1999). Experimental values were determined using equation (3-90).
T
K
|
Experimental data
|
Literature data
|
Deviation#
%
|
|
|
D/ cm3.min-1
|
|
|
303.15
|
7.550
|
13.278
|
21.4 -35.4
|
13.10
|
1.359
|
303.15
|
14.96
|
13.28
|
21.4 -35.4
|
13.10
|
1.374
|
303.15
|
20.23
|
13.271
|
21.4 -35.4
|
13.10
|
1.305
|
313.15
|
7.480
|
11.561
|
21.4 -35.4
|
11.80
|
-2.025
|
313.15
|
15.21
|
11.568
|
21.4 -35.4
|
11.80
|
-1.966
|
313.15
|
19.94
|
11.549
|
21.4 -35.4
|
11.80
|
-2.127
|
323.15
|
7.620
|
10.992
|
21.4 -35.4
|
10.90
|
0.844
|
323.15
|
15.09
|
11.009
|
21.4 -35.4
|
10.90
|
1.000
|
323.15
|
19.85
|
10.983
|
21.4 -35.4
|
10.90
|
0.761
|
Cyclohexane
|
303.15
|
7.510
|
8.199
|
21.4 -35.4
|
8.06
|
1.725
|
303.15
|
15.07
|
8.213
|
21.4 -35.4
|
8.06
|
1.898
|
303.15
|
21.53
|
8.184
|
21.4 -35.4
|
8.06
|
1.538
|
313.15
|
7.660
|
7.546
|
21.4 -35.4
|
7.40
|
1.973
|
313.15
|
15.14
|
7.539
|
21.4 -35.4
|
7.40
|
1.878
|
313.15
|
20.39
|
7.541
|
21.4 -35.4
|
7.40
|
1.905
|
323.15
|
7.480
|
6.836
|
21.4 -35.4
|
6.80
|
0.529
|
323.15
|
14.98
|
6.841
|
21.4 -35.4
|
6.80
|
0.603
|
323.15
|
21.43
|
6.839
|
21.4 -35.4
|
6.80
|
0.574
|
|
# Relative deviation, R.D., given by
| 


