3 MATERIALS AND METHODS
3.1 Water Hyacinth sampling site description
The water Hyacinth plants were collected in Nyabugogo swamp
which is located in capital city (Kigali) of the country. The part which is
shown as Nyabugogo swamp on the map is one which is not exploited by the
population for agriculture and is the one considered as natural wetland,
receiving wastewaters of Kigali City. Its surface area, according to CGIS, is
60.09 ha (CGIS, 2007).
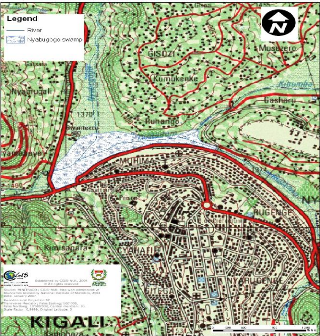
Figure 3.1: Topographic map showing the
location of Nyabugogo swamp and its influents. (Source: CGIS, 2007)
3.2 Methods
3.2.1 Description
The methodology developed in this research taken the approach
consisting in the laboratory pilot scale experiment. Major mechanisms of metal
removal were explained on basis of experimental results and available
information in the literature review. Adsorption and uptake, translocation and
foliar absorption tests were performed to assess the metal removal using water
hyacinth. Three replicates were done during the lab experiment.
3.2.2 Synthetic wastewater solution
preparation.
1 mol of ZnCl2 contains 1 mol of Zn (II) and also 1 mol of
K2Cr2O7 contains 2 mol of Cr(VI). Then knowing that the load of 1
mol ZnCl2 is 136.2 g ,1 mol K2Cr2O7 is 294 g, 1 mol Zn is 65.2 g and
2 mol of Cr is 2* 52 g = 104 g , we calculated the load of each salt to be
weighted and dissolved into 1 liter of aqueous solution. Thus for zinc, the
calculation has been done as follows: 1g * 136.28 g / 65.2 g = 2.1 g of ZnCl2
and for chromium we did it as follows: 1 g * 294g / 104 g = 2.8 g of
K2Cr2O7 .As we decided to prepare 100 ml of solution, 0.21g of ZnCl2
and 0.28 g of K2Cr2O7 were dissolved into 100 ml of solution. For 1
mg/l of Zn (II) and Cr(VI) preparation, we abstracted 1 ml from these 100 ml
and dilute up 1000 ml of solution. We did the same for 3 mg/l and 6 mg/l by
abstracting respectively 3 ml and 6 ml from the 100 ml of solution and dilute
up 1000 ml. The pH of the solution was then adjusted between 6 #177; 0.7 by
addition of dilute HNO3 or NaOH as required.
3.2.3 Experimental Procedures
The Water Hyacinth plants (Eichhornia crassipes) were
collected from Nyabugogo wetland in Kigali city, were rinsed with tap water and
distilled water to remove any epiphytes and insect larvae grown on plants. The
plants were placed in big plastic containers with water under natural sunlight
for several weeks to let them adapt to the new environment, then the plants
were selected and weighted by sensitive balance. The experimental set-up was
consisting in the use of small plastic container buckets of 16
cm of diameter and 14.5 cm in height. All experiments were run
in a batch system using a nutrient solution constituted by 500 ml of tap water
from the valley located at Butare near Pharmacopée centre, 500
ml of wastewater from the Nyabugogo wetland plus quantity of Ca(NO3)2 .4H2O,
NaNO3, NH4Cl, K2HPO4 respectively 20, 20, 20 and 40 mg. The fresh weight of the
plants in each bucket was measured by using sensitive balance before starting
each growing time: 1, 2 and 4 weeks.
A stock solution (1,000 mg/L) of Zn (II) (ZnCl2) and Cr (VI)
(K2Cr2O7) was prepared in distilled water, which was later diluted
as required. The plants were maintained in water supplemented by Heavy metals
by pouring a certain volume of the metals stock solution in order to get the
final concentration of 1, 3 and 6 mg/L of Cr and Zn respectively in different
plastic buckets containing water hyacinth plant in three replicates.
Plastic buckets with zinc and chromium concentrations without
water hyacinth plants served as control. Distilled water was added in order to
compensate for water loss through plant transpiration, sampling and
evaporation. Water samples were taken and pH measurements by pH meter were
taken every 60 minutes for the first day during 6 hours and for the following
days one sample after time period was taken during 1, 2, and 4 weeks of
exposure to metal solution. All samples were filtered using 0.45 um cellulose
acetate filters (wathman papers) and acidified with 5 drops of nitric acid
(HNO3 65%) in the laboratory for storage of water samples in volumetric flasks
(250 ml) before Atomic Absorption Spectrometer analyses.
The Figure 3.2 shows the plan view of laboratory experimental set
up developed during our research in National University of Rwanda, Faculty of
Sciences.

Figure 3.2: Plan view of experimental set
up.
After each test duration (1, 2 and 4 weeks), final fresh
weight for each water hyacinth plant was taken; plants were harvested for other
analyses. They were separated into petioles, roots and leaves and were analysed
for relative growth, metals accumulation, translocation ability,
bioconcentration factor (BCF) and adsorption on the outer surface of roots. For
adsorption, roots were immersed in EDTA-Na2 for metal desorption. All parts of
the plants were dried in drying oven at 105°C for 24 hours. In addition,
the metals remained in the solution were measured to assess the removal
potential of water hyacinth plants.
The figure 3.3 depicts different steps developed in the
laboratory for data collection and analyses.
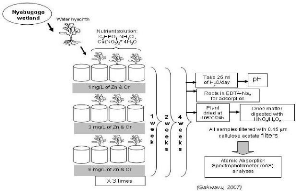
Figure 3.3: steps in lab experiment.
| 


