4.5.4 Discussions on adsorption mechanism
The adsorption ability of water hyacinth plants seems to be
different when zinc and chromium are compared. It was seen that for zinc 17.6%
of 1 mg/L was adsorbed by the water hyacinth plants, 6.1% of 3 mg/L was
adsorbed and the plants adsorbed 1.1% of 6 mg/L. Whereas for chromium, 9.0% of
1 mg/L, 36.4% of 3 mg/L and 54.6% of 6 mg/L were adsorbed on the roots of water
hyacinth plants.
4.6 Uptake mechanism
4.6 1 Uptake mechanism for zinc
The uptake mechanism was observed to identify which part of
water hyacinth plant contributes much in metal ions accumulation. The variation
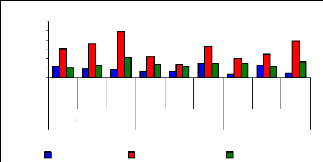
of uptake versus metal dosage for zinc are shown in Figure 4.16 and exhibited
linearity at the low level of exposure time (1 week for petioles and leaves);
however, the linearity trend could not be established with confidence for
leaves and roots for 1 and 4 weeks. The regression coefficients for zinc (II)
were found to be 0.6379 for 1 and 3 mg/L, 0.3195 for 3 and 6 mg/L and 0.3660
for 1 and 6 mg/L during all the experimental period. Thus, the uptake process
apparently followed an increasing trend with a linear increase of metal
concentrations in petioles for 1 week and 4 weeks but in 2 weeks, the pattern
of uptake changes. It was observed that petioles are important parts for metal
ions accumulation in water hyacinth plants
exposure time (wk) vs initial conc. (mg/L)
roots petioles leaves
Uptake mechanisms
0,6
0,5
0,4
0,3
0,2
0,1
0
Conc. (mg/Kg)
1 mg/L
1 mg/L
1 mg/L
3mg/L
3mg/L
6mg/L
3mg/L
6mg/L
6mg/L
4 weeks
2 weeks
1 week
Figure 4.16: Variations of uptake for zinc by the
plants
Figure 4.16 depicted the uptake of zinc (II), which shows to
be in normal distribution according to metal concentration, but it exhibits the
changes when exposure time increases. Thus, the present observations showed
that the extent of metals (Zn) uptake by plant was dependent on the
concentration of the metal in the solution as well as the length of exposure to
the plants.
4.6.2 Uptake mechanism for chromium
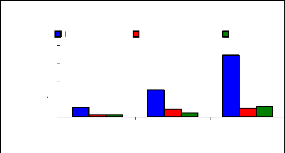
The figure 4.17 describes the uptake mechanism which
demonstrates the important part of water hyacinth plant in metal accumulation.
As seen from this figure, roots are important parts for chromium accumulation
in the plants. This show a difference with zinc, which was more accumulated in
petioles. This Figure 4.17 continues to show the behavior of chromium in plant
tissues and it is clear that roots are the important parts for the accumulation
of chromium in the water hyacinth plants. When chromium is mixed with zinc in
the same water samples, zinc is more mobile than chromium, so zinc will be
absorbed very quickly than chromium. Petioles come in second position in metal
uptake for 3 mg/L. The uptake is linear according to concentration for roots
and leaves but less for petioles.
dry weight
(mg/kg)
4
2
3
0
1
Uptake mechanism for chromium
roots petioles leaves
1 mg/L 3 mg/L 6 mg/L
Initial conc. (mg/L)
Figure 4.17: Uptake of chromium in plant tissues for
different initial concentrations
| 


