II.1.3 In vitro dissolution
1.3.1 Methods
· Preparation of dissolution
medium
Distilled water was used as dissolution medium.
· Calibration curve
Stock solution
30 mg of amoxicillin standard powder was accurately weighed
and dissolved into a required volume of dissolution medium to make a solution
having a concentration of 300mg/l, used as stock solution.
Standard solutions
5, 10, 15, 20, and 25 ml from the stock solution were
separately transferred to 25.0 ml volumetric flasks and diluted to volume using
dissolution medium. The resulting standard solutions had concentrations of 60,
120, 180, 240 and 300 mg/l. Absorbances of the above standard solutions were
spectrophotometrically measured at 272 nm.
A calibration curve (absorbance vs. amoxicillin concentration)
y = 0.003x + 0.0017 with a correlation coefficient (R2) of 0.9999
was constructed.
· Dissolution testing
Dissolution profiles were determined using the USP basket
method (Method 1) at a rotational speed of 100 rpm for capsules containing 250
mg, and using the USP paddle method (Method 2) at a rotational speed of 75 rpm
for capsules containing 500 mg.
Each of 6 capsules was placed inside a dissolution vessel
filled with 900 ml of dissolution medium maintained at 37 0.5°C. At
different time intervals (10, 20, 30, 40, 50 and 60 minutes) 5 ml of samples
were manually withdrawn, filtered, and analyzed spectrophotometrically at 272
nm for their amoxicillin concentration. Samples from 500 mg capsules were
diluted twice before analysis. The amount of the drug dissolved was calculated
by means of the above mentioned calibration curve.
1.3.2 Results
Table 1.2 shows the percentage dissolved within 60 minutes of
dissolution testing and Figure 1.1 the different dissolution profiles. Before
stability testing all drugs complied with the USP 24 dissolution requirements
(not less than 80% of the labelled amount should dissolve within 60 minutes).
The amount of drug released after 60 minutes of dissolution test was more than
90% for all formulations. The accelerated stability testing did not affect the
dissolution profiles; the percentage released remained within the USP 24 limits
for all formulations.
Table 1.2: Percentage amoxicillin dissolved within 60 minutes
of dissolution testing before and after 3 and 6 months of storage at 40°C
and 75% RH. USP requirements: more than 80 % released within 60 minutes.
Manufacturer
% of the labelled amount released
0 month 3 months 6 months
Elys chemicals (Elymox) 99.9
94.5 91.3
Labophar (Amoxyphar) 96.7
96.3 96.7
Dilam (Amoxysha 500) 104.2
102.9 97.7
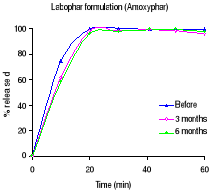


Figure 1.1: Dissolution profiles of amoxicillin formulations
before and after 3 and 6 months storage at 40°C and 75 % RH.
| 


