3.2.2 Results
The RSD was 0.47 and 0.24 % within a day and 1.51 and 1.29 %
within three days for sulfamethoxazole and trimethoprim, respectively; which
complies with the USP 24 requirements (RSD should be less than 2%). The
resolution factor R between sulfamethoxazole and trimethoprim was 8.02, which
means that they were well separated. As shown in Table 3.1, the S&R
formulation (Batrimox) failed to comply with USP 24 requirements in terms of
drug content for sulfamethoxazole (93 - 107 % of the labelled amount of
sulfamethoxazole and trimethorim).
There was no impact of stability testing on the drug content
for the Elys formulation (Unitrim), while the drug content of both
sulfamethoxazole and trimethoprim for the Labophar formulation (Bactiphar)
decreased.
Table 3.1 The
sulfamethoxazole and trimethoprim content (expressed as a percentage
of the labelled amount) before and after 6 months of stability testing at
simulated tropical conditions.
Manufacturer
% of the labelled amount per tablet
0 months 6 months
Sulfamethoxazole
Elys Chemicals (Unitrim) 97.1
94.6
Labophar (Bactiphar) 97.2
92.8
S&R pharmaceuticals (Batrimox)* 91.6
-
Trimethoprim
Elys Chemicals (Unitrim) 99.6
97.0
Labophar (Bactiphar) 98.1
84.8
S&R pharmaceuticals (Batrimox)* 97.4
-
* Not analyzed for stability testing because it failed the
dissolution test for both two
compounds immediately after purchase.
II.3.3 In vitro dissolution
3.3.1 Methods
· Preparation of dissolution
medium
98.64 ml of 37 % hydrochloric acid was diluted to 10.0 L with
distilled water. The resulting 0.1 N solution was used as dissolution
medium.
· Calibration curves of
sulfamethoxazole and trimethoprim
Based on the HPLC method, the calibration curves mentioned in
quantitative drug analysis were used for calculation of the amount of drug
released. The same mobile phase, the same standard solutions and the same
concentrations were used.
· Dissolution testing
Dissolution profiles were determined using the USP paddle
method (Method 2). Each of 6 tablets was placed inside a dissolution vessel
filled with 900 ml of dissolution medium maintained at 370.5°C stirred by
paddles rotating at 75 rpm. At 10, 20, 30, 40, 50 and 60 minutes 5 ml samples
were withdrawn, filtered, diluted 5 times and analysed for their contents of
sulfamethoxazole and trimethoprim by UV at 254 nm after chromatographic
separation.
Procedure
20 ul of each of the collected samples was injected onto the
HPLC system and the corresponding peak areas were recorded. The content of each
sample was calculated using the calibration curve.
3.3.2 Results
Table 3.2 shows the percentage drug dissolved and Figures 3.1
to 3.3 the dissolution profiles of different formulations analyzed. For
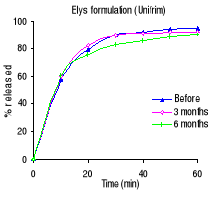
sulfamethoxazole the Elys formulation (Unitrim) complied with the USP 24
requirements (not less than 70% of sulfamethoxazole and trimethoprim labelled
amount should dissolve within 60 min), however the drug percentage released
decreased after 6 months of storage at 40°C/ 75% RH. Labophar formulation
(Bactiphar) released 45% of the drug, the S&R formulation (Batrimox)
released only 15%. Those last two formulations did not disintegrate completely
within 60 minutes. For trimethoprim, 90% of the labelled amount of Unitrim and
77.5% of Bactiphar were released within 60 min, which complies with USP 24,
while Batrimox failed (only 35.4 % was released).
Table 3.2 Percentage of sulfamethoxazole and
trimethoprim dissolved within 60 minutes of dissolution testing before, after 3
and 6 months of storage at 40°C and 75% RH. USP requirements: more than 70
% released within 60 minutes.
Manufacturer
% of the labelled amount per tablet
0 months 3 months 6 months
Sulfamethoxazole
Elys Chemicals (Unitrim) 98.0
94.2 77.0
Labophar (Bactiphar) 45.0
38.5 25.8
S&R pharmaceuticals (Batrimox) 15.0
- -
Trimethoprim
Elys Chemicals (Unitrim) 95.1
92.2 90.2
Labophar (Bactiphar) 77.6
47.4 32.5
S&R pharmaceuticals (Batrimox) 35.4
- -
Figure 3.1 In vitro dissolution profiles of sulfamethoxazole
and trimethoprim before stability testing
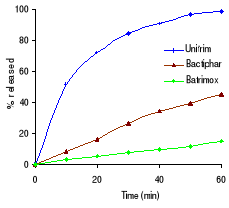
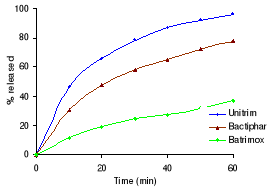
Figure 3.2 Dissolution profiles of sulfamethoxazole
formulations before and after 3 and 6 months storage at 40°C and 75 %
RH.
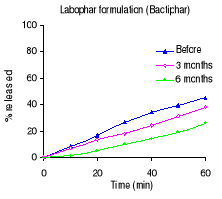

Figure 3.3 Dissolution profiles of trimethoprim formulations
before and after 3 and 6 months of storage at 40°C and 75 % RH.

| 


