2.5. Discussion
Small planktivorous fish such as sandeel, sprat, anchovy and
sardine are an important trophic group in marine ecosystems where they
facilitate the transfer of energy from lower trophic levels, the plankton, to
higher trophic levels such as large predatory fish and seabirds. Our analysis
of sandeels and sprats in the North Sea from 1960 to the end of the
21st century suggests that a continued warming of the North Sea will
cause the probability of occurrence of both species to decline as sea
temperature exceeds their thermal niches and the fish move northwards and
decline in abundance as a consequence. Encouragingly, our analyses of the
probability of occurrence of sandeel, sprat and snake pipefish predicted
changes in the abundance of these species that have already been observed at
the end of the 20th century and the first decade of the
21st century. Our model predicted that the probability of occurrence
of both the lesser sandeel and sprat should decreasse in the North Sea in two
phases, the first at the end of the 1980s and the second at the end of the
1990s and this coincides with two periods of intensification in warming in the
North Sea (Beaugrand et al. 2008). The NPPEN model also predicted a
transient increase in snake pipefish in the North Sea during the first decade
of the 21st century and this parallels an observed increase in their
abundance in the northeastern part of the North Atlantic, which was linked to
increased SST (Kirby et al. 2006), and also with reports of their
increased presence in the diet of North Sea seabirds during this time (Harris
et al. 2007). The transient increase in snake pipefish predicted by
NPPEN during 2000-2008 could therefore, arguably reflect the changing thermal
regime of the North Sea providing a temporary window of opportunity favouring
this species.
The lesser sandeel and sprat are important prey species for
several North Sea seabirds and changes in the abundance of these fish are
considered to influence the breeding success of birds such as guillemots
(Uria aalge, P.), kittiwakes (Rissa tridactyla, L.) and
Atlantic puffins (Fratercula arctica, L.) (Lewis et al. 2001;
Wanless et al. 2005). During the first half of the 21st
century, our model indicates that an increase or sustained probability of
occurrence of North Sea sprat could compensate any reduction in North Sea
sandeels (figure IV.2b). Furthermore, even by the end of the
21st century the reduction in the probability of occurrence of sprat
is more moderate along Scottish coasts of the North Sea, which might help
sustain seabird colonies albeit at lower numbers than at present. However, a
much more pronounced warming (see Scenario A2, and electronic supplementary
material, figure IV.S4) might precipitate the decrease in the probability of
sprat occurrence especially over coastal areas. Such a scenario would extend
the foraging journeys of adult seabirds, increasing the time that chicks are
left unattended at the nest, further affecting seabird breeding success
(Wanless et al. 2005). As kittiwakes are surface feeders unlike
guillemots, which are pursuit divers, a reduction in coastal sprat may
influence kittiwakes especially; guillemots less constrained in their foraging
depths may be less likely to encounter food limitation (Wanless et al.
2005).
In the North Sea, the reduction in the biomass of cod has
already been related to a decrease in the abundance of suitable plankton prey
during the fish-larval stage (Beaugrand & Kirby 2010a,b). During the second
half of the 21st century our analyses suggest that the breeding
success of North Sea seabirds may be affected similarly by a reduction in prey
occurrence. The North Sea supports a breeding population of about 20 seabird
species and among these kittiwakes and puffins may be highly sensitive to the
disappearance of lesser sandeels that comprise an important component of their
diets (Lewis et al. 2001; Poloczanska et al. 2004;
Frederiksen et al. 2005; Daunt et al. 2008). As the North Sea
warms due to hydroclimatic change it might be expected that warmer water,
southern species will colonise suitable habitats in the North Sea (Hiddink
& Ter Hofstede 2008). Indeed, colonisation by warm water species newly
recorded in the North Sea has already been observed in the both the plankton
and the benthos (Beaugrand et al. 2009; Lindley et al. 2010).
Bear and colleagues (Beare et al. 2004b) noticed that although
anchovies and sardines were very rarely observed during the period 1925-1994 in
the northern part of the North Sea, they became more prevalent after 1995.
Warmer water fish species have occurred in the North Sea before. For example
from 1900 to 1950, there was a commercial fishery in the region for Atlantic
bluefin tuna, Thunnus thynnus (MacKenzie and Myers 2007). In the
1930s, anchovies were exploited in the Dutch Wadden Sea (Boddeke &
Vingerhood, 1996). However, under neither climate change scenarios A2 nor B2
did the thermal regime of the North Sea encourage an increase in the European
sardine or anchovies within the Northern North Sea where the main seabird
breeding colonies currently occur. Anchovies might become exploitable by the
second part of the Century as it was in the 1930s (Boddeke & Vingerhood,
1996) but our model indicates it is unlikely to become abundant in the northern
part of the North Sea if warming follows Scenario A2 and B2. Our results
suggest therefore that neither the European anchovy nor the European sardine
will compensate for the adverse biological changes affecting the prey of North
Sea seabirds until the end of this century. However, it should be noted that if
warming becomes more intense, this figure will undoubtedly change.
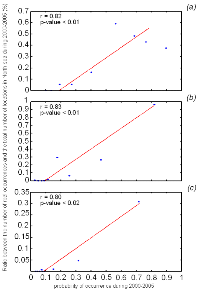
Figure IV.4 : Correlation (Spearman's rank
correlation) between the probabilities of occurrence modelled by NPPEN with the
percentage of the number of actual presence data points in the North Sea for
2000-2008 in the North Sea for (a) lesser sandeel,
(b) European sprat and (c) snake pipefish.
Only half of the data, not incorporated to the model, were used for validation.
If the preferred fish prey of North Sea seabirds declines in
abundance due to a northward movement constrained by their thermal niche, it is
worthwhile speculating as to what may happen to current seabird populations.
For example, climate-driven changes in the distribution of European sardine
have been the cause of latitudinal expansion of the Balearic shearwater, from
the French Biscay coast to southern UK coasts (Yésou 2003; Wynn et
al. 2007). While it seems unlikely that European anchovy, as well as
European sardine will occur in sufficient quantity in the North Sea to become a
prevalent prey at more northern latitudes for black-legged kittiwakes and
common guillemots, some northern seabird species could expand their range
southwards to take advantage of any increase in abundance of these two fish.
Southern movements of some species previously common in the Northern North Sea
have already been observed in response to North Sea warming. This hypothesis
implicates a large plasticity for other key environmental factors such as
temperature that can become a source of physiological stress even for these
endotherm species. How quickly North Sea seabirds may acclimate to a new diet
is uncertain since most seabirds have a highly specialised diet and it is
unclear whether they have sufficient plasticity to forage on alternative prey
species (Grémillet & Boulinier 2009).
There is strong evidence that the North Sea is a tightly
coupled ecosystem controlled by the hydroclimatic environment and influenced by
trophic interactions (Kirby et al. 2009; Beaugrand & Kirby 2010a,
b). Previously, we have shown how climate-induced changes in species
composition in the benthos, plankton and among fish have altered trophic
interactions in the pelagic food web to drive the North Sea towards a new
dynamic regime favouring jellyfish in the plankton and decapods and
detritivores in the benthos over commercial fisheries. The results we report
here extend the influence of hydroclimatic change to include the putative
consequences for the small pelagic fishes of the North Sea. In other marine
ecosystems where changes in top predators due to overfishing have freed the
small planktivorous pelagic fishes from top-down control, the subsequent
increase in their abundance has comprised trophic cascades that have influenced
plankton abundance and fish recruitment (Frank et al. 2005; Daskalov
et al. 2007). The decline in abundance of planktivorous fish in the
North Sea predicted by our model may therefore reinforce further the trophic
amplification of a climate signal already witnessed in the North Sea (Kirby
& Beaugrand 2009), and also extend this to the avian fauna providing the
most comprehensive example yet of the effects of climate-induced ecosystem
change. We remind that our model and projections are highly dependent on the
intensity of warming (Beaugrand et al. In press).
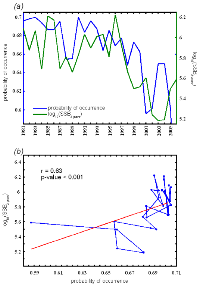
Figure IV.5 : Comparison
(a) and correlation analysis (Spearman's rank
correlation) (b) of long term changes of lesser
sandeel (two years old) spawning-stock biomasses (SSB, ICES 2010) and the
probabilities of occurrences modelled by the NPPEN in area IV for the period
1983-2006.
We used in this paper probable scenarios of SST change
(Intergovernmental Panel on Climate Change 2007b). More intense warming,
comparable to Scenario A1FI (IPCC 2007), could accelerate the biogeographic
movements and the trophic amplification in the North Sea ecosystems.
Acknowledgements
R.R.Kirby is a Royal Society Research Fellow. We thank the
Centre National de la Recherche Scientifique (CNRS) for financial support. We
thank Dr Maud Moison for helpful comments on the figures, and the IFREMER of
Boulogne-sur-Mer for helpful advice concerning IBTS surveys.
Supporting Information
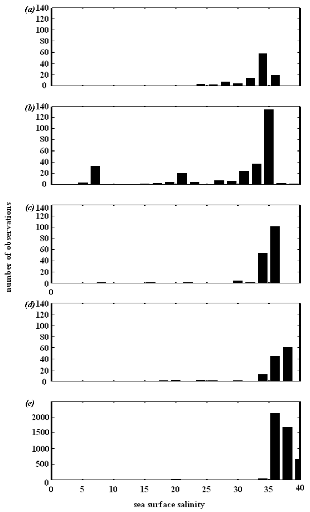
Figure IV.S1 : Salinity preferendum estimated
for (a) lesser sandeel, (b)
European sprat, (c) snake pipefish,
(d) European anchovy and (e) European sardine.
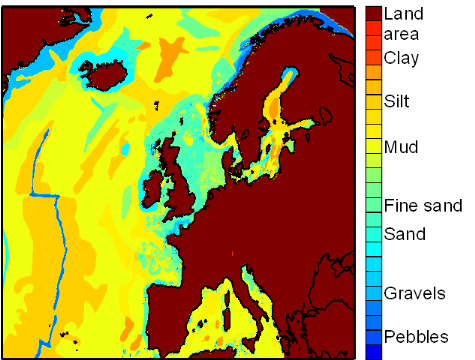
Figure IV.S2 : Spatial distribution of the
bottom-sediment type used for the estimation of lesser sandeel spatial
distribution.
Figure IV.S3 : Estimated probability of
occurrence using NPPEN for the time periods 1960-1969 (a),
2000-2008 (b), 2050-2059
(c) and 2090-2099 (d) in
the North Sea (bounding box) and adjacent seas for lesser sandeel.
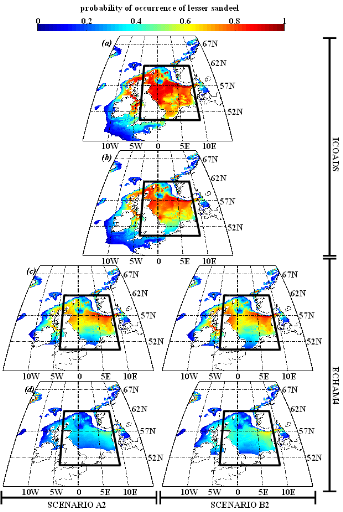
Figure IV.S4 : Estimated probability of
occurrence using NPPEN for the time periods 1960-1969 (a),
2000-2008 (b), 2050-2059 (c) and 2090-2099
(d) in the North Sea (bounding box) and adjacent seas for
European sprat
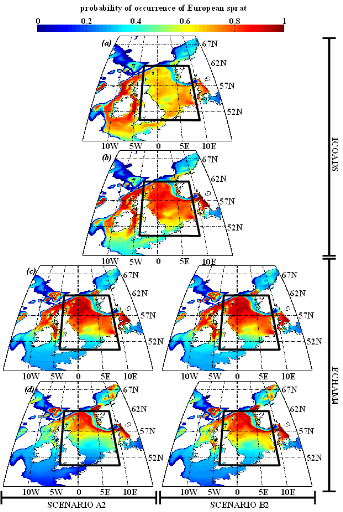
Figure IV.S5 : Estimated probability of
occurrence using NPPEN for the time periods 1960-1969 (a),
2000-2008 (b), 2050-2059 (c) and 2090-2099
(d) in the North Sea (bounding box) and adjacent seas for
snake pipefish.
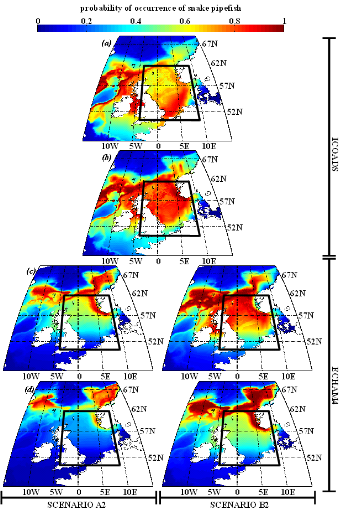
Figure IV.S6 : Estimated probability of
occurrence using NPPEN for the time periods 1960-1969 (a),
2000-2008 (b), 2050-2059 (c) and 2090-2099
(d) in the North Sea (bounding box) and adjacent seas for
European anchovy.
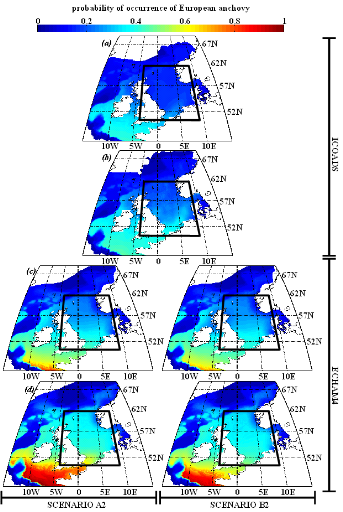
Figure IV.S7 : Estimated probability of
occurrence using NPPEN for the time periods 1960-1969 (a),
2000-2008 (b), 2050-2059 (c) and 2090-2099
(d) in the North Sea (bounding box) and adjacent seas for
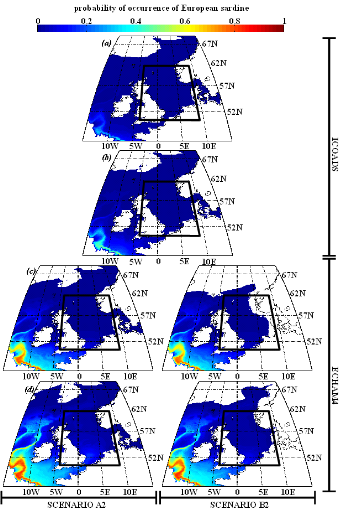
European sardine.
CHAPITRE V
Réchauffement climatique, Calanus
finmarchicus et la morue de l'Atlantique
| 


