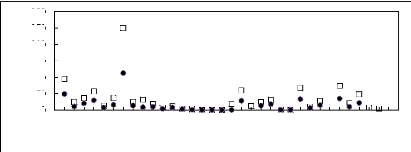
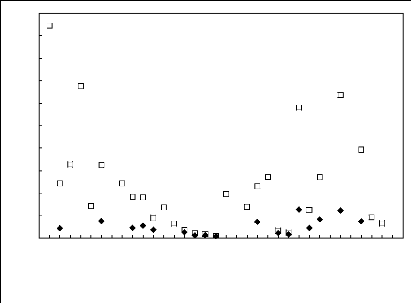
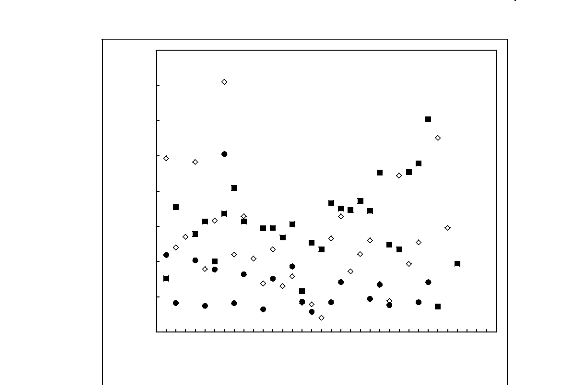
Figures 6-1 to 6-3 shows a comparison of limiting activity
coefficients of six solutes, each one representing a homologous series, in
fluorinated ionic liquids.
Infinite dilution activity
coefficient
250
200
300
150
100
50
0
0
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
Ionic liquids
Figure 6-1: Experimental infinite dilution
activity coefficients of (?) n-hexane and (?)
cyclohexane in various
fluorinated ionic liquids at 313.15 K.;1, [EMIM][BF4][1][2]; 2,
[EMIM]
[Tf2N] [3][5]; 3, [MMIM] [Tf2N] [4] ; 4, [BMIM][BF4] [6] ;
5, [BMIM] [Tf2N] [4] ; 6,
[BMIM][TfO] [9] ; 7, [MMPIM][BF4] [10] ; 8,
[EMMIM] [Tf2N] [3] ; 9, [HMIM][BF4] [12] ; 10,
[HMIM][PF6] [13] ; 11, [HMIM]
[Tf2N] [14][15] ; 12, [MOIM][BF4] [17] ; 13, [MOIM] [Tf2N] [16] ;
14,
[C16MIM][BF4] [18] ; 15, [3C6C14P][BF4] [19] ; 16, [3C6C14P] [Tf2N]
[19] ; 17,
[3C6C14P][(C2F5)3PF3] [20] ; 18, [3C1C4N] [Tf2N] [21] ; 19,
[BMPy][BF4] [22] ; 20, [BMPyrr]
[Tf2N] [16] ; 21, [Et3S] [Tf2N] [24] ; 22,
[Epy] [Tf2N] [25] ; 23, [3C6C14P][PF6] [19] ; 24, [3C8C1N]
[Tf2N]
[27]; 25, [EMIM][TfO] [28]; 26, [MOIM][PF6]
[29]; 27, [BMIM][SbF6] [30] ; 28,
[BMIM][PF6] [31] ; 29,
[EMIM][TFA] [32] ; 30, [HMIM][TfO] [33] ; 31, [BMPyrr][TfO] [34] ;
32,
[HMPyrr] [Tf2N] [35] ; 33 , [OMPyrr] [Tf2N] [35]
Superscripts on ionic liquids` abbreviations in this section,
as well as, in appendices F, G, H and I correspond to the following
publications used as references: [1] Ge et al. (2008a); [2] Foco et al. (2006);
[3] Heintz et al. (2002); [4] Krummen et al. (2002); [5] Deenadayalu et al.
(2005); [6] Zhou and Wang (2006); [7] Zhou et al. (2007) ; [8]Heintz et al.
(2005) ; [9]Domañska, U. and Marciniak, A., (2008a) ; [10]Wang et al.
(2008) ; [11] Ge et al. (2008b); [12]Letcher et al. (2003b) [13]Letcher et al.
(2003a) [14]Heintz et al. (2006a); [15]Letcher et al. (2005); [16]Kato et al.
(2005) [17]Heintz et al. (2005b) [18]Mutelet et al. (2007); [19]Domañska
et al. (2009); [20]Letcher and Reddy (2005); [21]Heintz et al. (2006b);
[22]Heintz et al. (2001); [23]Heintz et al. (2002); [24]Domañska and
Marciniak (2009); [25] Kato and Gmehling (2004); [26]Kato and Gmehling (2005);
[27]Gwala et al. (2010), [28]Olivier et al (2010a); [29]Olivier et al (2010b);
[30]Olivier et al. (2009c) ; [31]Shimoyama et al. (2008); [32]Domañska,
U. and Marciniak, A., (2007) [33]Yang et al. (2008) [35]Nebig et al. (2009);
[36] Möllmann and
Gmehling (1997); [37] Krummen et al. (2000); [38]Kossack et
al.(2008); [39]Dortmund Data Bank, DDB.
Infinite dilution activity coefficient
50
45
40
35
30
25
20
15
10
5
0
0
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
Ionic liquids
Figure 6-2: Experimental infinite dilution
activity coefficients of (?) hex-1-ene and () hex-1-
yne in various
fluorinated ionic liquids at 313.15 K; 1, [EMIM][BF4] [2]; 2,
[EMIM][Tf2N] [4][5];
3, [MMIM][Tf2N] [4] ; 4, [BMIM][BF4] [2][6] ; 5,
[BMIM][Tf2N] [4] ; 6, [BMIM][TfO] [9]; 8,
[EMMIM][Tf2N] [3] ; 9,
[HMIM][BF4] [12] ; 10, [HMIM][PF6] [13] ; 11, [HMIM][Tf2N] [14][15] ;
12,
[MOIM][BF4] [17] ; 13, [MOIM][Tf2N] [16] ; 14, [C16MIM][BF4] [18] ; 15,
[3C6C14P][BF4] [19] ;
16, [3C6C14P][Tf2N] [19] ; 17, [3C6C14P][(C2F5)3PF3]
[20] ; 18, [3C1C4N][Tf2N] [21] ; 20, [BMPyrr]
[Tf2N] [16] ; 21,
[Et3S][Tf2N] [24] ; 22, [Epy][Tf2N] [25] ; 23, [3C6C14P][PF6] [19] ; 24,
[3C8C1N]
[Tf2N] [27]; 25, [EMIM][TfO] [28]; 26,
[MOIM][PF6] [29]; 27, [BMIM][SbF6] [30] ;
29,
[EMIM][TFA] [32] ; 31, [BMPyrr][TfO] [34] ; 32,
[HMPyrr][Tf2N] [35] ; 33 , [OMPyrr][Tf2N] [35]
Chapter 6: Discussion
4
3.5
Infinite dilution activity coefficient
3
2.5
2
1.5
1
0.5
0
0
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
31
32
33
34
35
Ionic liquids
Figure 6-3: Experimental infinite dilution
activity coefficients of () ethanol, () benzene and
(?) acetone in various
fluorinated ionic liquids at 313.15 K.; 1, [EMIM][BF4] [2];
2,
[EMIM][Tf2N] [4]; 3, [MMIM][Tf2N] [4] ; 4, [BMIM][BF4] [6][7]
; 5, [BMIM][Tf2N] [8] ; 6,
[BMIM][TfO] [9] ; 7, [MMPIM][BF4]
[10][11] ; 8, [EMMIM][Tf2N] [3] ; 9, [HMIM][BF4] [2] ; 10,
[HMIM][PF6] [13]
; 11, [HMIM][Tf2N] [16] ; 12, [MOIM][BF4] [17] ; 13, [MOIM][Tf2N] [16] ;
14,
[C16MIM][BF4] [18] ; 15, [3C6C14P][BF4] [19] ; 16, [3C6C14P][Tf2N] [19]
; 17,
[3C6C14P][(C2F5)3PF3] [20] ; 18, [3C1C4N][Tf2N] [21] ; 19,
[BMPy][BF4] [22][23] ; 20, [BMPyrr]
[Tf2N] [16] ; 21, [Et3S][Tf2N] [24] ;
22, [Epy][Tf2N] [25][26] ; 23, [3C6C14P][PF6] [19] ; 24,
[3C8C1N][Tf2N]
[27]; 25, [EMIM][TfO] [28]; 26, [MOIM][PF6]
[29]; 27, [BMIM][SbF6] [30] ; 28,
[BMIM][PF6] [31] ; 29, [EMIM][TFA]
[32] ; 30, [HMIM][TfO] [33] ; 31, [BMPyrr][TfO] [34] ; 32,
[HMPyrr][Tf2N]
[35] ; 33 , [OMPyrr] [Tf2N] [35]
According to the above plots, activity coefficient values for
organic solutes in FILs follow the following patterns:
· Imidazolium, pyridinium, pyrrolidinium and
sulfonium-based fluorinated ionic liquids: n-alkanes > cycloalkanes >
alk-1-enes > alk-1-ynes > alcohols > alkylbenzenes > ketones
· Phosphonium-based fluorinated ionic liquids:
n-alkanes alk-1-enes alcohols > cycloalkanes > alk-1-ynes
> alkylbenzenes >
ketones
· Ammonium-based fluorinated ionic liquids:
No clear hierarchy is observable.
6.2.2. Effect of structure on IDACs of organic solutes
in Fluorinated Ionic Liquids, FILs
It would be misleading to conclude that the stated
hierarchies fully translate the behavior of all mixtures of fluorinated ionic
liquids and the solutes under consideration. These are merely general trends.
However, the only constant for all systems is that alkanes and ketones lead to
the largest and the smallest experimental infinite dilution activity
coefficient values respectively.
Linear alkanes are non-polar organic compounds. They interact
with ILs mostly through small range van der Waals forces which are weaker than
induced dipole-dipole interactions appearing in systems involving corresponding
alk-1-enes, alk-1-ynes and aromatic compounds. These compounds have delocalized
electrons that enhance polarisability. Increasing polarisability leads
naturally to smaller limiting activity coefficients. Infinite dilution activity
coefficient values of alcohols are even smaller due to their polar nature and
the presence of an electronegative oxygen atom which is likely to interact more
strongly with the positive charge of the ionic liquid cation. Infinite dilution
activity coefficients values for ketones are the smallest, an indication of
strong solute-solvent interactions. This can be attributed to the interaction
between the two pairs of electrons on the oxygen atom of the ketone with the
cation of the IL, as well as, between its positive pole and the ionic liquid
anion.
There are strong cation-anion coulombic interactions in
imidazolium and pyridinium-based ionic liquids due to the polarisability of
their molecules. Conversely, no delocalized electrons exist in phosphonium,
ammonium and sulfonium-based fluorinated ionic liquids. Association with
organic solutes is likely to be stronger with these solvents in comparison with
imidazolium and pyridinium-based ionic liquids as accommodating a solute will
not require overcoming the strong coulombic interactions and probably hydrogen
bonds. It follows that for the same solute, the limiting activity coefficient
value increases with the introduction of a polarisable ring in the cation of
the ionic liquid. Extending the alkyl chain of the solute generally weakens the
interactions between organic solutes and ionic liquids as infinite dilution
activity coefficients increase with increasing solute carbon number. This is
visible in Figures G-1 through G-83 of Appendix G which relate the infinite
dilution activity coefficient at 313.15 K to the carbon number of various
solutes in all investigated fluorinated ionic liquids. In the presence of
hydrogen bonds and coulombic forces that are common in ionic liquids,
long-chained ionic
liquids are probably too large and closely packed to
accommodate solutes. Exceptions include
the following systems: alkan-1-ols +
[BMIM] [Tf2N], alkan-1-ols + [3C6C14P] [(C2F5)3PF3] and
ket-2-ones + [C13C8N][Tf2N] . Their corresponding plots of the
natural logarithm of versus
carbon number are found in Figures G-56, G-59 and G-81
respectively. It is possible that lengthening the alkyl chain of an alcohol or
a ketone to a certain extent in these particular cases favours additional
attractive forces between the alkyl chain of the solute and the one of the
solvent through van der Waals interactions.
As far as the effect of the ionic liquid anion is concerned,
shape, polarity and size have to be taken into account to interpret infinite
dilution activity coefficient experimental results. Most polar ionic liquids
i.e. those containing [BF4] - and [TFA] - do not generally interact strongly
with the solutes due probably to strong intrinsic anion-cation coulombic
forces, as well as hydrogen bonds.



