CHAPTER TWO: LITERATURE REVIEW
Chapter overview
In order to assist in determining the significance of previous
similar works and understand the impact of the present study, this chapter
reviews the following:
· Ionic liquids in the chemical industry;
· Generalities on activity coefficient at infinite
dilution;
· Recent advances in the design of inert gas stripping
equipments.
Experimental and predictive methods for infinite dilution
activity coefficient determination are
outlined. Only the two experimental
techniques used in this work, gas-liquid chromatography
and the inert gas
stripping method are discussed. A closer look is taken at attempts made in
the
open literature to predict in ionic liquids. The most promising
predictive methods for
systems involving ionic liquids are discussed in terms of
accuracy and reliability. Since the objective of this study involves
understanding the effect of fluorinated ionic liquids` structure on their
ability to separate various mixtures and the construction of a dilutor cell,
the results obtained from previous scientific investigation in these regards
are reviewed. A treatment of ionic liquids in general and the fluorinated ones,
in particular, is provided.
2.1. Ionic liquids
2.1.1. Definition and structure
Ionic liquids are materials that are solely composed of
cations and anions, and melt at or below 100 oC (Endres and El
Abedin 2006). The most commonly found structures of ionic liquids are given in
figure 2-1.
2.1.2. History
In 1914, Paul Walden (1914) reported the physical properties
of the first useful ionic liquid, ethylammonium nitrate, which had a melting
point of 12 oC. It was synthesized by the reaction of concentrated
nitric acid with ethylamine. Thereafter, Hurley and Weir (1951) stated the
possibility of preparing room temperature ionic liquids by simply mixing and
warming 1- ethylpyridinium in the presence of aluminum chloride; Chum et al.
(1975), Robinson et al. (1979), Wilkes et al. (1982), Seddon et al. (1983) and
Appleby et al. (1986) directed extensive research works towards new aluminum
chloride-based ionic liquids in 1970s and 1980s. These ionic liquids did not
find many practical applications due to their hydroscopic nature which required
both preparation and handling to take place in an inert gas atmosphere. In
1992, water
and air stable 1-ethyl-3-imidazolium-based ionic liquids were
reported by Cooper and O`Sullivan (1992), as well as Zaworotko and Wilkes
(1992) in separate works. They developed imidazolium-based ionic liquids which
consisted of alternative anions: acetate ([CH3CO2]-), nitrite
([NO2]-), tetrafluoroborate ([BF4]-), trifluoromethane
sulfonate ([CF3SO3]-) and methylsulfate, ([CH3SO4]-).
Since then, new anions were incorporated in the structure of ionic liquids:
hexafluorophosphate ([PF6]-), biscyanamide ([N (CN)2]2-),
trifluoroacetate ([C2F3O2]-), sulfate ([SO4]2-),
hydrogensulfate ([HSO4]-), alkylsulfate ([R-SO4]-) ,
nitrate,([NO3]-). Contrary to chloroaluminates salts, these ionic
liquids can be prepared on the bench. However, they can absorb water from the
atmosphere, and they do not react with water. The development of more
hydrophobic ionic liquids started in the second half of the 1990s through the
work of Bonhôte et al. (1996). They reported the synthesis and
characterization of ionic liquids containing hydrophobic anions such as
trifluoromethane sulfonate ([CF3SO3]-), triflate
([OTf]-), tris (trifluoromethylsulfonyl) methanide {[C
(CF3SO2)3]-} and bis (trifluoromethylsulfonyl) imide. As scientific
research chemicals, ionic liquids were commercially available in 1999 (Letcher
2007). Nowadays, around 1018 ionic liquids have been predicted
(Endres and El Abedin 2006) and could be synthesized by combining different
ions like the ones shown in figure 2-1.
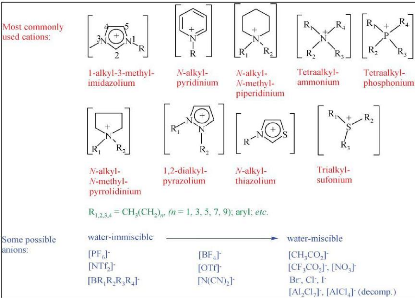
Figure 2-1: Structure of ionic liquids
(Plechkova and Seddon 2008)
2.1.3. Properties of ionic liquids
It is because of their advantageous properties that ionic
liquids attracted much attention from the scientific and industrial spheres.
The most interesting properties of these compounds are listed below:
a) Extremely low vapour pressure: they are eco-friendly. (Welton
1999, and Wasserscheid and Keim 2000);
b) Low or reduced flammability hazards (Fox et al. 2008);
c) Tunable properties (Rogers and Seddon 2003);
d) Excellent solvation properties for a variety of organic and
inorganic compounds (Rogers and Seddon 2003);
e) High electric conductivities (Trulove and Mantz 2003);
f) High thermal stability (Dupont 2004);
g) Wide liquid range (Huddleston et al. 2001);
h) Wide electrochemical window (Shröder et al. 2000).
Though ionic liquids are generally known as safe and benign
compounds, some of them are volatile (Earl et al. 2006) and others are
flammable (Smiglak et al. 2006).
| 


