Abstract
Caelifera is one of the largest and most diverse group of
insects, and they are the dominant Orthoptera in agriculture ecosystems. We
present here an inventory of Acridid fauna of the agricultural ecosystems in
the Mzab valley (Septentrional Sahara, Algeria). Grasshoppers were sampled with
quadrats in Béni Isguen, Ghardaïa and El-Atteuf, and we have
expressed the species richness, sampling effort and relative abundance. The
method used is that of quadrats sampling. The results revealed the presence of
27 species, divided into three families, Acrididae, Pyrgomorphidae and
Tetrigidae. Among these families, we found that Acrididae are most diverse with
six subfamilies. The subfamily Oedipodinae with 10 species was the most
abundant, while the subfamily Tetriginae was the least abundant (represented by
a one species). The value of the diversity index showed that the cultivated
area of Béni Isguen is the most favorable for the development of many
Caelifera. The abundance of grasses and low intensity of agricultural activity
in this environment favors the presence of Acridid. We found that the Acridid
community depends mainly on the nature and richness of vegetation cover, the
intensity of agricultural activity and the type of irrigation.
Keywords: Grasshopper, agricultural ecosystem,
biodiversity, North Africa, Sahara
Introduction
Currently, the number of Orthoptera species described
worldwide is about 28159 species (Eades et al. 2018). Acridoidea is
the notable superfamily of the suborder Caelifera having 518 species (Yadav
et al. 2017). Furthermore, 241 species of Orthoptera Acridomorpha
Dirsh 1975 have been listed for Morocco, Algeria, Tunisia and the Western
Sahara (Louveaux et al. 2013). Algeria has about 114 Orthoptera,
including 98 Caeliferans. A significant part of Algeria is inhabited by
grasshoppers. These insects are one of important pests since the onset of
agriculture. They can cause great damage to agricultural production when
climatic conditions are conductive to their multiplication. Therefore, it is
necessary to have comprehensive knowledge of all grasshopper species that occur
in an area (Benkenana & Harrat 2009). Given the danger of these Acridids,
several studies have been carried out all over the world, including Algeria. We
cite among others those of Chopard (1943), Dirsh (1965), Zergoun (1991, 1994),
Doumandji-Mitiche et al. (2001), Ould El Hadj (2002), Maurel (2008),
Benfekih et al. (2011), Guendouz-Benrima et al. (2011),
Moussi et al. (2011, 2014, 2018), Louveaux et al. (2013),
Massa (2013) and Defaut (2017). However, the grasshopper fauna of Algeria in
general and of the Sahara in particular needs more studies; because the only
species that are well-studied are the gregarious and economically important
species such as the migratory locust Locusta migratoria (Linnaeus,
1758), the desert locust Schistocerca gregaria (Forskål, 1775),
and the Moroccan locust Dociostaurus maroccanus (Thunberg 1815). The
objectives of these investigations of the grasshoppers in agriculture
ecosystems of Mzab valley
18 Accepted by Petr Kocarek: 7 Feb. 2019;
published: 28 Feb. 2019
ACRIDID DIVERSITY IN AGRICULTURE ECOSYSTEMS J. Insect
Biodiversity 009 (1) (c) 2019 Magnolia Press
·
19
were to (i) investigate grasshopper species composition of
three studied sites, (ii) found the abundance and diversity of grasshopper
species in studied sites, (iii) demonstrate the effect of vegetation cover, the
intensity of agricultural activity and the type of irrigation on the richness
and diversity of Caelifera.
Materials and methods
Study area
The study was conducted in three different localities of the
Mzab valley : N'tissa (Béni Isguen), Touzouz (Ghardaïa) and
El-Djaoua (El-Atteuf). The Mzab valley is located in the Septentrional Sahara
(northern Algerian Sahara). Its elevation is 483.63 m above mean sea level,
between 32° to 33° north latitude and between 3° to 4° east
longitude (Fig. 1).
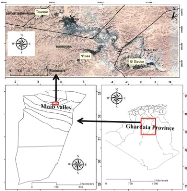
Figure 1. Geographic location of the surveyed
sites in the Mzab valley, Septentrional Sahara (Mzab valley, Ghardaïa
province, Algeria).
The choice of studied sites is based on the variability in
three criteria: vegetation cover, agricultural intensity and irrigation. At
each study site, the floristic composition was determined by visual survey of a
randomly placed quadrat of 500 m2. For each plant, the cover was
estimated by calculating of the surface area occupied by the orthogonal
projection of the aerial part, according to Duranton et al. (1982).
Agricultural intensity was quantified by the percentage of agricultural crops.
Humidity was quantified by the frequency and amount of irrigation. The study of
the Acridid fauna was conducted in three different localities: N'tissa
(Béni Isguen), Touzouz (Ghardaïa) and El-Djaoua (El-Atteuf).
Béni Isguen (S1): latitude 32° 44' North, and
longitude 3° 65' East. The surface area of the site is about 2 hectares.
The vegetal cover is mainly composed of date palms (Phoenix
dactylifera), fruit trees (Citrus sinensis (L.) Osbeck and
Vitis vinifera L.) and vegetable crops (Cucurbita siceraria
(Molina) Standl., Cucurbita maxima Duchesne, Citrullus
lanatus (Thunb.) Matsum. & Nakai and Cucumis melo L.) cover
25%. Also there was an abundance of weeds, 30% is covered by Cynodon
dactylon (L.) Pers., Setaria verticillata (L.) P. Beauv,
Polypogon monspeliensis (L) Desf., Hyparrhenia hirta (L)
Stapf and Stipagrostis plumosa (L.) Munro ex Anderson. The intensity
of agricultural activity is low; irrigation is irregular.
Ghardaïa (S2): latitude 32° 51' North and longitude
3° 59' East. The surface of the site is 6 hectares. Among the plants, we
find date palm (Phoenix dactylifera L.), citrus fruits (Citrus
sinensis (L.) Osbeck), olive trees (Olea europaea L.), and
vegetable crops (Cucurbita siceraria (Molina) Standl., Cucurbita
maxima Duchesne, Citrullus lanatus (Thunb.) Matsum. & Nakai
and Cucumis melo L.) cover 50%. The Poaceae cover 15% (Cynodon
dactylon (L.) Pers., Setaria verticillata (L.) P. Beauv and
Polypogon monspeliensis (L) Desf.) and are located under the trees.
The intensity of agricultural activity is average. Irrigation is normal and is
done by drip system.
El-Atteuf (S3): latitude 32° 44' North, and longitude
3° 72' East. The surface of the site is 4 hectares. The vegetation is
dominated by forage crops (Medicago sativa L. and Hordeum vulgare
L.) which cover 50%, date palm (Phoenix dactylifera L.) and vines
(Vitis vinifera L.) cover 30% and a few weeds (Cynodon dactylon
(L.) Pers. and Setaria verticillata (L.) P. Beauv) cover 5%. The
intensity of agricultural activity is strong. Irrigation is by sprinkler
irrigation for forage crops (alfalfa and barley) and drip irrigation for other
crops (fruit and vegetables).
20
· J. Insect Biodiversity
009 (1) (c) 2019 Magnolia Press ZERGOUN ET AL.
This Saharan region is characterized by a dry and cold climate
in winter and dry, hot summers. Precipitation in the Mzab region are weak and
irregular. The main rains occur in autumn. Rainfall is very rare during the
whole year (less than 70 mm per annum). During summer, the air relative
humidity falls to as low as 10%, resulting in a strong evaporation. However, in
winter it rises up to 48.6%. In the Mzab region, there are two types of winds:
north-west dominant sand-laden winds and south-north dominant hot and dry winds
(named sirocco). The highest wind speeds occur in the month of April and are in
the range of (14.4 m/s). The climatic data were collected over the period 2007-
2016 from the Office of Algerian meteorology. We used the ombrothermic diagram
of Bagnouls & Gaussen (1953), which defines empirically a dry month as when
the total precipitation (in mm) recorded during a month is lower or equal to
double the average temperature (in °C) of the same month. For the area
considered (2007-2016), the dry season lasts 12 months (Fig. 2).
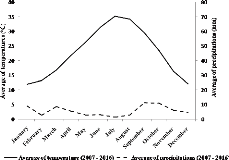
Figure 2. Graphical representation of
T°-P Ombrothermic diagram (2007-2016) in the Mzab valley, Ghardaïa
province, Algeria (National Office of Meteorology, Ghardaïa).
Sampling and identification
Methods for Orthoptera sampling are numerous and very diverse
(Lamotte & Bourlière 1978; Voisin 1980, 1986; Gillon 1974). In the
present study, the selected method is that of quadrats, the most frequently
reported method used for biodiversity studies of terrestrial ecosystems. The
method used for surveying grasshoppers in this study is reported by Gardiner
et al. (2002). The size of the quadrats used in the survey was 25
m2 (5×5 m). Ten quadrats were positioned at random in one
hectare plot at each study site. The corners of each quadrat were marked using
poles without the observer disturbing the grasshoppers within by casting
shadows. Each plot at the study sites was surveyed to ascertain grasshopper
abundance and species richness. Samplings were carried out very early in the
morning, between 7:00 a.m. and 9:00 a.m. in summer, and between 9:00 a.m. and
11:00 a.m. in winter, when the insects were still immobilized on the ground.
For each site, the quantitative measure of acridian density was carried out
using the quadrats method. The total number of individuals belonging to each
species was counted in quadrats (25 m2). The counting in the
quadrats were repeated ten times for each site and day of sampling. Density
from each site has been reported as grasshopper per 100 m2.
Acridid were collected by sweeping a hand net. A standard net
of 40 cm in diameter having a depth of 60 cm with a 90 cm long wooden handle
was used for sample collecting. Grasshoppers were collected in the different
localities once a month in each ecosystem from January to December 2017. The
collected specimens were killed using ethyl acetate and properly stretched,
pinned and labeled. Specimen identification up to the species level was done
with the aid of the taxonomic keys of Chopard (1943); Dirsh (1965), and also
Catalogue and keys of the Acridomorpha (Insecta, Orthoptera) from North West
Africa (Louveaux et al. 2018).
Data analysis
Relative abundance of grasshopper species was calculated as
the number of individuals of species i relative to the total number of
individuals of all species collected at each site.
Richness, number of individuals of species, species diversity
and evenness (Shannon-Weiner index and evenness index) of the grasshopper
community was evaluated with the help of statistical package Past 3.4 (Hammer
et al. 2014). Species richness estimates with 95% confidence intervals
for the estimators Sest (analytical) and Chao1 Classic based
ACRIDID DIVERSITY IN AGRICULTURE ECOSYSTEMS J. Insect
Biodiversity 009 (1) (c) 2019 Magnolia Press
·
21
on 100 randomized samples for the data of Caelifera sampled
was applied. Estimated species richness was calculated with the program
EstimateS 9.1.0 (Colwell 2013). An Analysis of Similarities ANOSIM (Clarke
1993) was used to test if the differences in structure and composition of
grasshopper communities in each site and throughout the year were significant.
The Bray-Curtis coefficient was used as a distance measure. We used
Kruskal-Wallis non-parametric one-way analysis of variance to test for
differences among the disturbance categories in grasshopper species richness
and grasshopper diversity. Except as otherwise noted, all statistics were
performed using R version 2.15.3 (R Core Team 2013). All data transformations
and graphs were done in Excel. P values of 0.05 or smaller were interpreted as
significant.
Results
During 36 surveys in the 3 studied sites of the Mzab valley, a
total of 5255 Acridid specimens, representing 27 species were recorded in 360
quadrats. Identification of these specimens showed that 23 species belonged to
the Acrididae, 3 to the Pyrgomorphidae and one to the Tetrigidae. Twenty three
Acrididae grasshoppers could be separated into 6 subfamilies and 13 genera,
while the 3 Pyrgomorphidae could be grouped into one subfamily and two genera.
The Tetrigidae is represented by a single sub-family and a single genus. A list
of short-horn grasshopper species found in the 3 studied habitats during our
12-month sampling period is presented in Table 1.
Table 1. Inventory and relative abundance (%)
of short-horn grasshopper species of agriculture ecosystem in three localities
from the Mzab valley (Septentrional Sahara, Algeria).
|
Family
|
Subfamily
|
Species
|
S1
|
S2
|
S3
|
|
Tetrigidae
|
Tetriginae
|
Paratettix meridionalis (Rambur, 1839)
|
0.57
|
-
|
1.18
|
|
Acrididae
|
Acridinae
|
Acrida turrita (Linnaeus, 1758)
|
4.21
|
2.23
|
4.27
|
|
|
Truxalis nasuta (Linnaeus, 1758)
|
1.42
|
0.56
|
-
|
|
Calliptaminae
|
Calliptamus barbarus (Costa, 1836)
|
0.06
|
-
|
-
|
|
Eremogryllinae
|
Notopleura saharica Krauss, 1902
|
0.62
|
0.45
|
-
|
|
Eyprepocnemidinae
|
Heteracris adspersa (Redtenbacher, 1889)
|
0.91
|
-
|
-
|
|
|
Heteracris annulosa Walker, 1870
|
3.69
|
3.44
|
3.22
|
|
|
Heteracris harterti (Bolivar, 1913)
|
1.48
|
-
|
-
|
|
|
Heteracris littoralis (Rambur, 1838)
|
1.82
|
1.01
|
1.44
|
|
|
Heteracris minuta (Uvarov, 1921)
|
0.51
|
-
|
-
|
|
Gomphocerinae
|
Ochrilidia filicornis (Krauss, 1902)
|
1.48
|
2.53
|
2.30
|
|
|
Ochrilidia geniculata (Bolivar, 1913)
|
3.58
|
4.51
|
9.39
|
|
|
Ochrilidia gracilis (Krauss, 1902)
|
17.68
|
16.82
|
15.96
|
|
|
Ochrilidia harterti (Bolivar, 1913)
|
4.77
|
9.88
|
-
|
|
Oedipodinae
|
Aiolopus puissanti Defaut, 2005
|
1.88
|
2.03
|
3.74
|
|
|
Aiolopus simulatrix (Walker, 1870)
|
2.67
|
1.47
|
2.56
|
|
|
Aiolopus strepens (Latreille, 1804)
|
12.28
|
7.87
|
10.84
|
|
|
Acrotylus longipes (Charpentier, 1845)
|
2.10
|
1.21
|
2.23
|
|
|
Acrotylus patruelis (Herrich-Schäffer, 1838)
|
13.13
|
14.89
|
14.32
|
|
|
Hilethera aeolopoides (Uvarov, 1922)
|
0.51
|
1.52
|
0.06
|
|
|
Locusta migratoria (Fabricius, 1781)
|
-
|
0.15
|
-
|
|
|
Morphacris fasciata (Thunberg, 1815)
|
9.61
|
10.43
|
15.11
|
|
|
Sphingoderus carinatus (Saussure, 1888)
|
-
|
0.56
|
-
|
|
|
Sphingonotus azurescens (Rambur, 1838)
|
-
|
1.11
|
-
|
|
Pyrgomorphidae
|
Pyrgomorphinae
|
Pyrgomorpha cognata Krauss, 1877
|
7.50
|
9.27
|
8.01
|
|
|
Pyrgomorpha conica (Olivier, 1791)
|
6.93
|
8.05
|
5.32
|
|
|
Tenuitarsus angustus (Blanchard, 1836)
|
0.57
|
-
|
-
|
|
03
|
08
|
27
|
100
|
100
|
100
|
S1: N'tissa (Béni Isguen), S2: Touzouz (Ghardaïa),
S3: El-Djaoua (El-Atteuf).
Overall, the species found regularly in the surveys are also
the most abundant in terms of frequency. Species richness was higher at sites 1
and 2 compared to site 3. Béni Isguen was the richest site with 24
species, followed by Ghardaïa (21 species). El-Atteuf was the least rich,
with only 16 species.
Non-parametric methods for estimating the true species
richness indicated that the inventory for each habitat was complete. The
accumulation curves for all species stabilized by the 12th survey
for the three sites (Fig. 3).
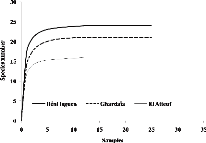
22
· J. Insect Biodiversity
009 (1) (c) 2019 Magnolia Press ZERGOUN ET AL.
Figure 3. Sample-base rarefaction curve (with
95% confidence intervals) for the estimated species richness in the three study
areas (Béni Isguen, Ghardaïa and El-Atteuf).
Species richness was higher at sites 1 and 2 compared to site
3. At Béni Isguen and Ghardaïa, presence of adventitious vegetation
has favored the increased total richness of Caelifera. Béni Isguen
seemed to be the richest site with 24 species; it is followed by Ghardaïa
with 21 species. El-Atteuf was the least rich, with only 16 species. The
relative abundance of Acridid population among the localities surveyed (Table
1) indicated that populations of Acrotylus patruelis, Aiolopus
strepens, Morphacris fasciata, Ochrilidia gracilis and
Pyrgomorpha cognata, were dominant at the three localities with
16.86%, 14.14%, 11.51%, 10.20% and 8.31% frequencies, respectively. The
Shannon-Wiener index confirms these results, with the highest value (2.65 bits)
reported at Béni Isguen and the lowest value (2.43 bits) recorded at
El-Atteuf (Table 2). In addition, El-Atteuf seems to be more balanced (E =
0.87), followed by Ghardaïa and Béni Isguen with 0.84 and 0.83,
respectively. The diversity index shows a somewhat variable result in different
seasons. The diversity index was highest between March and October and lowest
during winter in the three sites studied. On the other hand, the equitability
index was highest in winter. The lowest equitability index was observed during
late summer and autumn.
Table 2. Different ecological indicators
applied to short-horn grasshopper species of agriculture ecosystem in three
localities from the Mzab valley (Septentrional Sahara, Algeria).
|
Months
|
Béni Isguen
|
|
|
Ghardaïa
|
|
|
|
El-Atteuf
|
|
|
|
S
|
N
|
H
|
E
|
S
|
N
|
H
|
E
|
S
|
N
|
H
|
E
|
|
January
|
11
|
39
|
2.23
|
0.84
|
8
|
30
|
1.94
|
0.87
|
9
|
34
|
1.96
|
0.79
|
|
February
|
16
|
97
|
2.42
|
0.70
|
10
|
97
|
2.14
|
0.85
|
9
|
45
|
2.18
|
0.92
|
|
March
|
17
|
138
|
2.51
|
0.72
|
12
|
162
|
2.10
|
0.68
|
9
|
89
|
1.97
|
0.80
|
|
April
|
21
|
163
|
2.69
|
0.70
|
14
|
102
|
2.24
|
0.67
|
11
|
115
|
2.18
|
0.80
|
|
May
|
19
|
150
|
2.60
|
0.70
|
13
|
63
|
2.26
|
0.74
|
14
|
120
|
2.28
|
0.70
|
|
June
|
19
|
149
|
2.71
|
0.79
|
15
|
221
|
2.28
|
0.65
|
15
|
165
|
2.44
|
0.76
|
|
July
|
20
|
162
|
2.50
|
0.61
|
17
|
412
|
2.46
|
0.69
|
16
|
266
|
2.55
|
0.80
|
|
August
|
21
|
149
|
2.54
|
1.61
|
20
|
323
|
2.50
|
0.61
|
15
|
247
|
2.41
|
0.74
|
|
September
|
20
|
228
|
2.63
|
0.69
|
20
|
268
|
2.51
|
0.61
|
13
|
261
|
2.20
|
0.69
|
|
October
|
20
|
198
|
2.64
|
0.70
|
17
|
170
|
2.54
|
0.75
|
13
|
96
|
2.24
|
0.72
|
|
November
|
11
|
116
|
2.03
|
0.69
|
15
|
113
|
2.25
|
0.63
|
11
|
76
|
2.18
|
0.80
|
|
December
|
11
|
70
|
1.97
|
0.65
|
7
|
13
|
1.82
|
0.88
|
4
|
8
|
1.32
|
0.93
|
|
Total
|
24
|
1759
|
2.65
|
0.83
|
21
|
1974
|
2.55
|
0.84
|
16
|
1522
|
2.43
|
0.87
|
S: Richness, N: number of individuals of species (adults and
nymphs), H: Shannon-Weiner index, E: Evenness index.
The grouping of grasshopper responded differently to each site
(ANOSIM = R: 0.1123, P = 0.006; Fig. 4). Pairwise comparisons showed that the
grouping of grasshoppers on each agricultural ecosystem was significantly
different. The community composition of grasshopper responded differently to
each month (ANOSIM R = 0.152, P = 0.001; Fig. 5). Pairwise comparisons showed
that the community composition of Acridid on each month was significantly
different in each site.
There were not found any significant differences for Caelifera
abundance between the three sites (Kruskal-Wallis test; x2 =
0.82153; df = 2; p-value = 0.6631). Contrarily, the Caelifera richness in the
three agricultural ecosystems were
ACRIDID DIVERSITY IN AGRICULTURE ECOSYSTEMS J. Insect
Biodiversity 009 (1) (c) 2019 Magnolia Press
·
23
significantly different (Kruskal-Wallis test; x2 =
9.2183; df = 2; p-value = 0.00996). The same was true for the diversity between
the three habitats (Kruskal-Wallis test, x2 = 7.4135, df = 2,
p-value = 0.02456).
Total grasshopper density varied during the study period.
Higher densities were reached in July (164.8 individuals/100 m2) at
Ghardaïa and (106.4 individuals /100 m2) at El-Atteuf, for
Béni Isguen the highest value is recorded in August (99.6 individuals
/100 m2). Lower densities in December at El-Atteuf and
Ghardaïa, with a grasshopper density of 3.2 individuals /100 m2
and 5.2 individuals /100 m2, respectively (Fig. 6).

Figure 4. Analysis of similarities (ANOSIM)
plot showing dissimilarity between and within three sites. Bold horizontal bar
in the box indicates median; bottom of the box indicates 25th
percentile; top of the box indicates 75th percentile; whiskers
extend to the most extreme data point, which is no more than the range (i.e.
1.5) times the interquartile range from the box; width of the bar is directly
proportional to sample size.

Figure 5. Analysis of similarities (ANOSIM)
plot showing dissimilarity between and within Months. Bold horizontal bar in
the box indicates median; bottom of the box indicates 25th
percentile; top of the box indicates 75th percentile; whiskers
extend to the most extreme data point, which is no more than the range (i.e.
1.5) times the interquartile range from the box; width of the bar is directly
proportional to sample size.
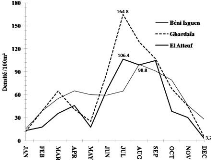
Figure 6. Monthly variation in grasshopper
population density recorded during the study period in the three studied sites
(Béni Isguen, Ghardaïa and El-Atteuf).
24
· J. Insect Biodiversity
009 (1) (c) 2019 Magnolia Press ZERGOUN ET AL.
Discussion
A total of 27 species of grasshoppers were collected from
different habitats of the Mzab region. Louveaux & Ben Halima (1987) listed
140 Caelifera species for entire Algeria. In this case with 27 species, the
Mzab valley is occupied by 19.28% of Algerian Caelifera-fauna. All the
grasshoppers collected are classified under three families Acrididae,
Pyrgomorphidae and Tetrigidae. Family Acrididae was dominant with 23 species of
Acridids grouped under 13 genera and six subfamilies, amounting to 85.18% of
collected species. The second most abundant family was Pyrgomorphidae with
three genera and one subfamily, which contributed 11.11% (3 species) of the
total species, while the Tetrigidae was represented by only one species
(3.70%). The trend of numerical distribution of different grasshopper families
recorded in the present study is similar to the observations of Belhadj et
al. (2014), who also reported that Acridid grasshoppers were the most
abundant group followed by Pyrgomorphidae and Tetrigidae in three sites at
Ouargla oasis (Algeria). Similar to Zergoun (1994), the species Calliptamus
barbarus has been reported only once in the Mzab valley, and has not been
found since. Three species were recorded for the first time in Mzab valley:
Hilethera aeolopoides and Morphacris fasciata (in the three
studied sites), and Notopleura saharica (in Béni Isguen and
Ghardaïa).
The differences between the three environments are related to
three parameters including, in order of importance: the nature and richness of
vegetation cover, intensity of agricultural activity, and type of irrigation.
The abundance of grasshopper species (27) was highest in Béni Isguen
(24) and Ghardaïa (21). The El-Atteuf studied site was represented by the
smallest number of species (16). The presence of adventitious vegetation has
favored the increased total grasshopper richness at Béni Isguen and
Ghardaïa. According to Paulraj et al. (2009), grasses were the
most common habitat for grasshoppers. This finding is consistent with our
results. Gramnivorous grasshoppers such as Ochrilidia gracilis,
Acrotylus patruelis and Morphacris fasciata were most
abundant, probably because our sites are very rich in grasses. According to
Jaulin (2009), the presence of an herbaceous layer is therefore essential for
most species of Orthoptera encountered.
The majority of Acridid species were found in the herbaceous
layer, such as Ochrilidia gracilis, Aiolopus strepens,
Acrida turrita, Pyrgomorpha cognata and Morphacris fasciata,
or naked soil such as Acrotylus patruelis, Tenuitarsus angustus
and Sphingonotus azurescens, on which they find their food and
can lay eggs. This is in agreement with the results obtained by Benjelloun
et al. (2014). El-Atteuf site has a low rate of grass cover due to
permanent agricultural work with sprinkling irrigation. All of these parameters
mean that this environment is less populated by Acridid.
As more and more samples are examined, it becomes harder and
harder to find species not already counted, so the slope of the curve gets less
and less steep as the sampling effort continues. With the sampling effort
performed at each habitat, all species accumulation curves reached an
asymptotic phase (Fig. 3)
Upon analysis of sites based on species abundance matrix using
Bray-Curtis dissimilarity, the agricultural ecosystem was significantly
different. These could be due to the difference in habitat such as vegetation
cover, agricultural intensity and micro-climate; point out that the vegetation
cover especially Poaceae, seems to be a principal cue for grasshoppers to
select a habitat. This is also mentioned by Zergoun (1994). Higher humidity and
increased agricultural activity were associated with decrease in abundance of
grasshoppers, while greater vegetation cover (especially of Poaceae), was
correlated with higher abundance of Acridids. A similar pattern was observed in
composition of grasshoppers in each month, which reinforces the argument that
humidity, agricultural intensity as well as vegetation cover play an important
role in determining grasshopper assemblages in the study area. These factors
also playing role in the feeding sites selection by Acridids. On the other
hand, Oedipodinae are only rarely observed on the vegetation. According to Otte
(1984) Oedipodinae usually are abundant only in areas with relatively sparse
ground cover.
We found, that habitat management had a significantly
different influence on species diversity and species richness according to its
intensity in studied agricultural ecosystems. A high diversity and richness of
Caelifera indicates a very low level of agricultural activity. Contrary to what
was expected, monthly abundance of grasshoppers did not vary significantly
among the sites, although, total abundance tended to be higher in Ghardaïa
and Béni Isguen than in El-Atteuf.
The value of the diversity index shows that the cultivated
area of Béni Isguen is the most favorable for the development of many
Caelifera. Indeed, it is characterized by an abundance of grasses such as
Cynodon dactylon and Setaria verticillata. In addition, the
low intensity of agricultural activity in this environment favors the presence
of Acridid. The site El-Atteuf is less diversified; this is due to frequent
weeding and therefore a low presence of grasses. In addition, sprinkler
irrigation creates a humid microclimate which limits the development of
grasshoppers. Equitability does not varying much in the three cultivated
environments; it means that the species are equally distributed. It is noted
that the El-Atteuf site has a slightly higher evenness than the other sites,
despite its low diversity. These results are
ACRIDID DIVERSITY IN AGRICuLTuRE ECOSYSTEMS J. Insect
Biodiversity 009 (1) (c) 2019 Magnolia Press
·
25
most consistent with (Frontier 1982) reasoning. According to
this author, a community comprising a small number of relatively abundant
species and the others are rare, appears to be less diversified than a
community comprising in total the same number of species, but with more
equitably distributed frequencies.
Densities were higher in disturbed sites El-Atteuf and
Ghardaïa. Similar results were observed by Cigliano et al. (2002)
in Benito Juarez County, Southern Pampas, Argentina where disturbed habitats
resulted in increased overall grasshopper densities. The monthly variations in
the densities show larger amplitudes at El-Atteuf than at Ghardaïa and
Béni Isguen. Grasshopper total density was affected by seasonal
variation. The greatest density was observed between April and October. The
maximum densities were recorded in summer. Thus, weather variables explained
the spatio-temporal variation of the grasshopper density. This result does not
agree with those registered in the Southern Pampas, Argentina, where
grasshopper density was not affected by seasonal precipitation and temperature
(Wysiecki et al. 2011). On the other hand, densities tended to
increase in hot and dry conditions in our study.
Grasshopper species richness and diversity in three sites vary
significantly among the months. Contrary to what was expected, total abundance
in month did not vary significantly.
The site El-Atteuf, which has undergone a strong agricultural
activity during our sampling, is less rich than the other two sites. Weeding
destroys a large part of the spontaneous plants such as Poaceae. In fact, the
herbaceous layer strongly contributes to the species richness of Acridids.
Thus, the agricultural activity has a negative impact on Acridids. In contrast,
some species such as Oedipodinae, commonly abundant on bare soil can be favored
in this area. Sprinkler irrigation has a negative effect on Acridid richness.
Knowing that most Acridids prefer warm and sunny places, this type of
irrigation will create a moist environment, and consequently, the exclusion of
many species that will seek drier environments. Generally, agricultural
practices such as irrigation and weeding (decline in Poaceae) often lead to the
destruction of species habitats, and cause a general decline in Acridid
biodiversity as reported by Tscharntke et al. (2005). It is
recommended that similar studies be conducted on a large scale in other regions
in order to fully assess the grasshopper fauna of the Algerian Sahara.
| 


