|
Academic year 2011- 12
Republic of Tunisia
Ministry of Higher Ministry of youth and
sports
Education and Scientific Research
UNIVERSITY OF SFAX
High Institute of Sport and Physical Education of Sfax
Master degree in Sciences and technique of physical and sport
activities. (Specialty: Biological Sciences)
MASTER'S THESIS
EFFECT OF RAMADAN FASTING ON THE SYMPATHOVAGAL BALANCE
THROUGH A STUDY OF HEART RATE VARIABILITY
By:
Mohamed EL Amine FANNANI
Supervisors:
Dr. Imed LATIRI
Dr. Mohamed Ali SAAFI
THANKS
This work has been completed thanks to many people whom I
cannot list here exhaustively. I am, however, deeply grateful to all those who
have supported, helped, encouraged and mentored me.
I want to express my gratitude to Mr. Imed LATIRI, Assistant
Professor of Higher Education at the Faculty of Medicine of Sousse, for having
accepted the charge of my direction. I thank him very sincerely for his
kindness, patience and invaluable help.
I would like to thank Dr. Mohamed Ali SAAFI, a university
hospital assistant at Sahloul Hospital in Sousse, who helped and directed me a
lot. May he find in this work the expression of my deep gratitude and deep
respect.
I would also like to thank Professor Zouhaïer TABKA for
the welcome he has given me in his research unit. Without his support, this
work would never have succeeded.
My compliments to all my teachers of the High Institute of
Sport and Physical Education of Sfax.
Thank you very much to the members of the jury who were kind
enough to have accepted to evaluate this work.
Finally a big thank to all the volunteers who participated in
this study.
DEDICATION
To my dear parents Hedi and Najet Nabiha
For their great affections and their sacrifices approved
throughout my university studies. May they find in this work the expression of
my eternal love and my infinite gratitude.
To my sisters Wafa and Wiem and her husband Riadh
For their encouragement, their support, their affection, with all
my wishes of happiness and success.
To all the members of my family and all my friends.
That they find in this work, my recognitions for their patience,
their kindness and their pleasant company.
Table of Contents
Introduction 10
Part I : literature review 13
I- Ramadan, change of habits and sports performances 14
I.1. Changing of style of life during the month of Ramadan 14
I.2. Effect of fasting Ramadan on body weight 16
I.3. Effects of Ramadan Fasting on Sports Performance 16
II - The heart rate variability 20
II.1. Autonomic nervous system 20
II.1.1. The sympathetic nervous system 22
II.2. Influence of autonomic nervous system on heart rate 23
II.3. Cardiac variability study 23
Part II : Material and Methodes 29
I. Participants 30
II. Experimental procedures 30
II.1. The Wingate test 31
II.2 Recording of heart variability 31
III. Statistical analysis 33
Part III : Results 34
I. Anthropometric characteristics of the sample 35
II. The Wingate test 35
II.1. Average power (P ave) 35
II.2 Peak power (Ppic) 36
III. Effect of fasting Ramadan on the autonomic nervous system
37
III.1. Effect of Ramadan fasting on the sympathetic system 39
III.2Effect of Ramadan fasting on the parasympathetic system
41
III.3. Effect of Ramadan fasting on sympathovagal balance 45
III.4. Effect of fasting Ramadan on the durations RR (ms) 46
Part IV : Discussion 48
I. The effects of fasting Ramadan on body weight 49
II. The effects of Ramadan fasting on Wingate test performances
49
III. The effect of fasting Ramadan on the heart rate variability
51
5
Conclusion 55
Bibliography 57
Figure 10: Average(#177; SD) of the RRs (ms) recorded during the
second, fourth week and before
Ramadan (n = 9) 46
List of Figures
Figure 1: Average (#177; SD) mean wingate power (W) values
recorded during the second, fourth week
and before Ramadan (n = 9) 36
Figure 2: Average (#177; SD) of the Peak (W) Wingate ratings
recorded during the second, fourth week
and before Ramadan (n = 9) 36
Figure 3: Average (#177; SD) LF (ms2) values
recorded during the second, fourth week and before
Ramadan (n = 9) 39
Figure 4: Average (#177; SD) LF (nu) values recorded during
the second, fourth week and before Ramadan
(n = 9) 40
Figure 5: Average (#177; SD) of RMSSDs (ms) recorded during
the second, fourth, and before Ramadan (n
= 9) 41
Figure 6: Average(#177; SD) of PNN50 (%) recorded during the
second, fourth week and before Ramadan
(n = 9) 42
Figure 7: Average(#177; SD) of the HF (ms2) values
recorded during the second, fourth week and before
Ramadan (n = 9) 43
Figure 8: Average(#177; SD) of the HF (nu) values recorded
during the second, fourth week and before
Ramadan (n = 9) 44
Figure 9: Average(#177; SD) values (LF / HF) recorded during
the second, fourth week and before
Ramadan (n = 9) 45
List of Tables
Table I: Index of human heart rate variability in the
frequency and time domains and their
approximate matches of 24-hour records 26
Table II: Average #177; SD of anthropometric characteristics
of subjects during the second week, the
fourth week and before Ramadan (n = 9) 35
Table III: Average #177; SD of wingate test parameters during
the second and fourth week of Ramadan
and after Ramadan (n = 9) 35
Table IV: Average (#177; SD) of parameters of analysis of
cardiac variability in the supine position 37
Table V: Average (#177; SD) of parameters of analysis of
cardiac variability while standing 37
Table VI: Average (#177; SD) of the parameters of the analysis
of the cardiac variability during the
effort 38
Table VII: Heart rate averages (bpm) recorded before, in the
middle and at the end of the month of
Ramadan (n = 9). 47
List of Photos
Photo 1: Achievement of the Wingate test 31
Photo 2 and 3: Recording resting heart rate variability 32
List of Abbreviations
B R: Before Ramadan
CMJ: Countermovement jump
Cm: Centimetres
ECG: Electrocardiogram
MVF: Maximum voluntary isometric force
R 4: End Ramadan
G: Grams
Hz: Hertz
HF: High frequency
Kg: Kilograms
LF: Low frequency
M: Meters
Ms: Milliseconds
R1: Second week of Ramadan
R2: Fourth week of Ramadan RF: Ramadan fasting
RR: Time interval between the two successive peaks of the R-waves
of the ECG
NN50: Number of successive RR intervals greater than 50 ms
Nu: Normalized
PNN50: Percentage of successive RR interval differences greater
than 50 ms
RMSSD: Square root of squared differences of successive RR
intervals
Ppic: Peak power
Pave: Average power
Pmax: Maximum power
SNA: Autonomic Nervous System
SDNN: Standard deviation of the RR interval over the entire
recording period
SNV: Vegetative nervous system
ULF: Ultra low frequency
VLF: Very low frequency
HRV: heart rate Variability
VO2 max: maximal oxygen consumption
Introduction
T
11
this holy month, Muslims who reached the required age
(puberty) should not eat, drink, smoke or engage into sexual intercourse, from
dawn to sunset. As the lunar Muslim calendar counts eleven to twelve days
shorter than the solar calendar and no intercalation, Ramadan shifts each year
and gradually changes from one season to another (kadri et al.,
2000).
During the last two decades, numerous studies tried to
evaluate the effects of Ramadan fasting (RF) on both physiological level and
clinical level. Their results showed that during this holy month, people change
their daily habits, promoting a more sedentary lifestyle because they tend to
stay up late, watching TV, praying or reading the holy Coran (Afifi et
al., 1997). There is also a tendency to eat, at night, food and
beverages that are higher in calories than those consumed during other months
(Ziaee et al., 2006).
It was also revealed that the occurrence of irritability,
headaches, and lack of sleep were distinguishably highlighted while fasting, in
addition of an increased fatigue during the whole month. General exhaustion,
reduced vigilance, low sense of well-being and weakened cognitive functions are
the results of changes in eating habits and sleep deprivation (Kadri et
al., 2000, Leiper et al., 2003, Roky et al., 2004). This may as well
explain the increase of vehicle accidents and the inflow of Muslims to medical
services during this month (Langford et al., 1994, Shanks et al.,
1994).
The physiological and clinical effects of Ramadan have been
the subject of many studies for many years (Zinker et al., 1990,
Ramadan et al., 1999, Bouhlel et al., 2006). Body weight reduction was
confirmed in some studies (Husain and al., 1987, Hallak & Nomani
1988, Ramadan and al., 1999), other work founded weight gain during
this month (Frost and Pirani, 1987, Yucel and al., 2004, Siddiqui et
al., 2005), while other authors note no significant changes in body
weight during this month (El Ati et al., 1995, Finch et al. 1998,
Ramadan 2002).
According to some studies, results showed an increase in fat
oxidation during sub-maximal exercise, which becomes moderate towards the end
of Ramadan (Bouhlel and al., 2006, Stannard and Thompson 2007).
Only an increase of urea and uric acid in serum was frequently
reported which could be attributed to dehydration during this month
(Ramadan et al., 2002, Roky et al., 2004).
The interest of cardiac function has been addressed by some
studies. The effect of fasting on the increase in heart rate caused by exercise
remains ambiguous. Indeed, some authors have
12
reported no effect of fasting on heart rate (Whitley
et al., 1998, Montain et al., 1991); while other authors have reported
a decrease in heart rate during fasting even during exercise (Husain R
et al., 1987, Nieman et al., 1987, Lam et al., 1996, Zoladz et al.,
2005).
It is obvious that the influence of Ramadan in various
clinical and physiological areas has aroused the interest of scientists these
last decades. Therefore, we propose to study the effects of fasting Ramadan on
Heart Rate Variability before and during the wingate test done by young
footballers aged from 15 to 16 years. The aim of this study is to:
· Recognize the effect of RF on
anaerobic sports performance through a laboratory test (the Wingate test).
· Identify the effects of RF on the
activity of the autonomic nervous system through the analysis of heart rate
variability.
Part I : literature review
14
I- Ramadan, change of habits and sports performances
I.1. Changing of style of life during the month of Ramadan
The major changes in the rhythm of life in Ramadan
essentially affect the time of food intake and sleep (Chaouachi et al.,
2008, Maughan etal., 2008a, Leiper et al., 2008). This is associated
with a change in the total amount of energy consumed (Angel &
Schwartz. 1975). Indeed, two or three meals (usually two), are taken
between dawn to sunset but, Since Ramadan is a lunar month, it is not fixed to
a specific Gregorian month. Thus, the period of time during which food and
water intake is permitted is variable, long in winter and short in summer in
the northern hemisphere of the terrestrial globe (Sobhani et al.,
1997). Indeed, the usual dietary practice is to consume a large meal
just after sunset and a lighter meal before dawn (the Shour) (Roky et
al., 2001, Ibrahim et al., 2008). It has been reported, moreover, a
greater variety of food consumed during Ramadan compared to the rest of the
year (Hallak & Nomani, 1988). Frost and Pirani (1987)
showed that energy intake was higher during Ramadan compared to post
Ramadan (3680 kcal / day versus 2425 kcal / day). In contrast, other studies
(Ziaee et al., 2006, Bouhlel et al., 2008, Chennaoui et al., 2009)
have shown a decrease in daily calorie intake during the month of
Ramadan. The calorie deficit negatively influences aerobic performance
(Aragon & Vargas, 1993). As for anaerobic performance,
they are negatively affected by caloric deficits (Mc Murray et al.,
1991). In contrast, other studies have shown that the total food
intake over a 24-hour period during Ramadan remains the same compared to the
control period despite the decrease in the frequency of food intake in the
nycthemeron (El Ati et al. 1995, Afifi, 1997, Taoudi et al., 1999,
Beltaifa et al., 2002, Souissi et al., 2007b, Meckel et al., 2008).
The large amount of food consumed in the evening is likely to prevent
the onset of falling asleep (Waterhouse, 2010). In fact, the
rat experiment found a significant correlation between the number of calories
consumed during the meal and the duration of the next sleep (Danguir
& Nicolaidis, 1979).
However, for Reilly and Waterhouse (2007),
this relationship is less obvious in humans. To say that sleep during
this month is also disturbed by the change in daily habits is banal. Muslims do
indeed tend to watch later by spending their time watching television, praying
or reading (Afifi, 1997 ; BaHammam, 2005), which delays sleep
and reduces its duration (Bogdan et al., 2001).These changes
in rhythm imposed by Ramadan thus affect the circadian system. If daytime fast
times are interrupted by periods of sleep (such as naps), the normal (sleep /
wake) cycle will be disrupted (Reilly & Waterhouse,
2007).
15
It should be noted that most studies that looked at sleep
during Ramadan used questionnaires to evaluate the characteristics of sleep of
individuals who work, study, or train themselves to fast during the month of
Ramadan. Some of them have shown that the number of hours of sleep decreases
during the month of fasting (Chennaoui et al., 2009). On the
other hand, other studies (Zerguini et al., 2007, Meckel et al., 2008,
BaHammam et al., 2010) have not reported a reduction in the number of
hours of sleep per day during Ramadan compared to before Ramadan. A study by
Roky et al. (2001) on sleep architecture during Ramadan using
polysomnography showed a delay in sleep and a reduction in sleep duration,
which can induce partial sleep deprivation (Roky et al 2001, Leiper et
al., 2008, Chennaoui et al., 2009). It has been shown, too,an increase
in daytime sleepiness during the fasting month, which was associated with
changes in circadian rhythm, central temperature, and fasting metabolic changes
(Roky et al., 2003). Vigilance decreases between
10h:00 am and 12h:00 am during this month of Ramadan
especially during the last week. It increases, however, around
14h:00, probably because of the absence of lunch which usually leads
to falling asleep (El Kalifi, 1998). Recall that sleep is
initiated by the drop in central temperature (Murphy & Campbell,
1997). So-called thermogenic factors such as nocturnal food intake
(Smith et al., 1994), pre-sleep light exposure (Dijk
et al., 1991), and nocturnal sport (Mizuno et al. 1998)
are likely to delay sleep. The delay in sleep and the reduction in
sleep duration observed during Ramadan can lead to partial sleep deprivation
(Roky et al., 2001), which can influence athletic
performance.
Few studies have investigated the effects of total sleep
deprivation on aerobic performance (VanHelder & Radomski, 1989).
The latter authors claim that the most recent studies support the
effect of sleep deprivation on aerobic performance. As for the literature on
the impact of partial sleep deprivation on anaerobic performance is very rich
.Symons et al., (1988) found that 60 hours of sleep
deprivation had no effect on Peak Power (PP), mean power (MP),fatigue index,
and blood lactate concentrations measured during a Wingate test.
In addition, Souissi et al., (2008) found
that peak power (Ppic) and mean power (MP) were lower due to sleep deprivation
at the end of the night compared with sleep deprivation at the beginning of the
night compared to a reference night. In the same study, the authors noted that
the anaerobic powers recorded in the morning following sleep deprivation at the
beginning or end of the night, and those recorded at night following the sleep
deprivation at the beginning of the night were not modified by compared to the
reference night.
16
In another study based on sleep limitation (imposed bedtime
and wake-up time respectively at 3:00 am and 7:00 am), Mougin et al.,
(1996) did not observe any variations in maximum speed, peak and and
mean powers and blood lactate concentrations measured in a 30 sec Wingate test
against a reference night. In short, partial sleep deprivation does not seem to
affect muscle power. Forcibly, muscle strength seems to be little affected by
partial sleep deprivation. (Bambaeichi et al., 2005) have
shown that the maximum isometric force of knee extensors is not altered by
partial sleep deprivation.
However, it should be noted that lack of sleep itself has
little direct effect on muscle activity, but it has an indirect effect on
physical performance because of changes in mental performance, motivation and
coordination (Reilly & Waterhouse, 2009). So, we could say
that the reduction of sleep gives rise to a fall in performance when the
exercises require sensorimotor coordination or cognitive processes. The risks
of this decrease in performance increase both with the strength of sleep
deprivation and the importance of the neuronal component of the exercise in
question (Mougin et al., 1996). In this vein, it has been
shown that motivation is lost in cases where the exercises are repeated or in
training sessions where several tasks are repeated to achieve a targeted goal
(Waterhouse, 2010).
I.2. Effect of fasting Ramadan on body weight
Experiments on the effect of fasting during the month of
Ramadan on body weight have yielded divergent results. In fact, some studies
have revealed a decrease in body weight during this month (Husain et
al., 1987, Hallak & Nomani, 1988, Ramadan et al., 1999, Roky et al., 2001,
Bouhlel et al. 2006, 2008, Ziaee et al., 2006, Chaouachi et al., 2008, Maughan
et al., 2008a). Other studies have not reported significant changes in
body weight during the fasting period (El Ati et al., 1995, Finch et
al., 1998, Ramadan 2002, Souissi et al., 2007, Zerguini et al. 2007, Meckel et
al., 2008, Chennaoui et al., 2009). While some studies have shown
weight gain during this month (Frost & Pirani, 1987, Yucel et al.,
2004, Siddiqui et al., 2005). These divergent conclusions are
explained by differences in daily habits (dietary and other), occupations and
also in the social and geographical environment that can influence the energy
balance (Meckel et al. , 2008).Thus different factors explain
the divergence of the conclusions drawn by the researchers.
I.3. Effects of Ramadan Fasting on Sports
Performance
The effect of Ramadan fasting on sports performance has been
the subject of very varied and diverse studies. The conclusions drawn are,
moreover, divergent. However, the exact mechanisms responsible for the declines
in these performances are not clearly defined
17
(Barret al., 1999, Maughan, 2010). Indeed,
multiple and interlocking factors related to the athlete, himself, the nature
of the sport, the weather conditions that the athlete faces, the schedule and
the duration of the exercise to be performed, influence the effect of fasting
on sports performance. Maughan et al (2010) reported that the
effect of Ramadan fasting differs from one sporting discipline to another and
from one athlete to another. They also noted that there are several difficult
situations that the athlete faces when he fasts during Ramadan. These include
endurance events in hot or humid weather, multi-day events, or late-night
events, or the example of athletes who face challenges during competitions
lasting more than 30 minutes at high temperature. As for Armstrong et
al., (1985), they put forward the example of the competitions that are
scheduled at the end of the day of the month of Ramadan. They note that fasting
athletes may be hypo hydrated before the start of competition, which is likely
to lead to a loss of performance. Warned, some athletes take before the
competition, certain provisions to avoid and limit this hypo hydration.
However, the inability to ingest fluids during exercise remains unresolved and
the risk of loss of performance remains high (American College of
Sports Medicine, 2007).
Burke et al., 2006; Shirreffs et al., 2006
also pose the physiological problem of the inability to replace sweat
losses and ingest carbohydrates to begin the process of replenishing muscle
glycogen in the immediate recovery period at a competition. That said, other
factors that are responsible for the fall in athletic performance among Ramadan
athletes have also been identified .In addition, the time of day when the test
is performed, the physical condition of the subjects and the measures taken can
be determining factors (Reilly and Waterhouse, 2007). In
addition, although there is a decrease in athletic performance during Ramadan,
it is unclear whether there is a systematic decline in physiological variables
related to exercise (Waterhouse, 2010). On the other hand, and
to our knowledge, research studies the impact of Ramadan fasting on disciplines
where exercise protocols are the most difficult ( Intermittent high intensity
exercises requiring physical and cognitive skills such as marathon, high-level
football match, tennis matches, road cycling competitions, etc.) especially in
hot conditions are very rare or nonexistent (Maughan et al.,
2010).
On aerobic-dominated performance, Sweileh et al.
(1992) showed that maximum oxygen uptake (VO2 max) decreases during
the first week of Ramadan, then returns to values before Ramadan at the last
week of this month. This can be explained by a physiological adaptation linked
to fasting. In addition, Sweileh et al. (1992) also found a
lower resting VO2 in the afternoon during Ramadan. This indicates, according to
Meckel et al., (2008) a strategy for
18
conserving energy reserves. It should be noted that fasting
has also been associated with decreased venous return, resulting in lower
sympathetic tone, leading to a reduction in blood pressure, heart rate and
cardiac output (Stokholm et al., 1991; Al Suwaidi et al., 2006).
These physiological changes can negatively influence the ability of
physical work and promote the deterioration of sports performance
(Meckel et al., 2008).Similarly, Meckel et al., (2008)
observed that Ramadan fasting leads to a reduction in aerobic
endurance performance (3000m run) in young footballers (14-16 years old). These
authors did not indicate the time of day or the season during which the tests
were performed. Chennaoui et al., (2009) also noted, in
middle-aged runners who train 6 to 10 times a week, a decrease in maximum
aerobic speed on the first and the third week of Ramadan in September compared
with before Ramadan. The duration of fasting was around 13 hours a day. These
authors explained the drop in performance observed by maintaining the same
volume of training during Ramadan despite the constraints associated with it
(sleep deprivation, caloric restriction and fatigue).
Similarly, Kirkendall et al., (2008) noted
that the endurance of young footballers assessed by the shuttle run test
established by Léger and Lambert, 1982, was affected
during the second week of Ramadan but this capacity is restored towards the end
of month of fasting. The authors explain the divergence of their results with
the previous results (Zerguini et al., 2007, Meckel et al., 2008)
by the change of the living conditions during Ramadan since in their
study, the young footballers resided together and were under control throughout
the investigation, which is not the case for the other two studies. In
addition, the intensity and duration of the training sessions and the quality
of sleep were not changed during Ramadan. In contrast, Chaouachi et
al., (2009) found no change in maximal oxygen uptake or heart rate
peak recorded in high-level judokas that maintained high training intensity and
volume during Ramadan. These researchers explained their results by the fact
that Ramadan fasting-induced metabolic constraints, concomitant with
maintaining a high training intensity, have little effect on the performance of
high-level athletes.
In terms of anaerobic performance, Bigard et al.,
(1998) showed that the month of Ramadan affects muscular strength and
endurance. Indeed, the maximum voluntary isometric force (MVF) of the elbow
flexors decreases as of the first week of Ramadan. In addition, the MVF of knee
extensors and muscular endurance at 35% and 70% of the MVF of the knee
extensors and elbow flexors decreased at the end of the month. Similarly,
Souissi et al., (2007) showed that maximum muscle powers
(Pmax) recorded during the strength / velocity test were lower
19
during Ramadan than before Ramadan (for tests conducted in the
afternoon). On the other hand, during the Wingate test, Souissi et al.
(2007) found that the peak powers (Ppic) recorded around
17h:00 and around 21h:00 were lower during the month of
fasting and during the second week compared to before Ramadan. Moreover, the
average powers recorded around 17h:00 and 21h:00 during
the same test were lower during the fourth week of Ramadan than before Ramadan,
while they were unchanged during the second week of the month of fasting
compared to the control session Ramadan.
In addition, Meckel et al., (2008) observed
that Ramadan fasting in results in a reduction in speed endurance (6 x 40m) and
countermovement jump (CMJ) performance in young footballers (14-16 years). On
the other hand, performances in sprint (40m) and agility (4x10m shuttle race)
were not affected during this month. The experiments made by Zerguini
et al. (2007) showed that the performances recorded during the
vertical Jump tests were not affected while those recorded during the 20 m
sprint test, the dribbling speed test and the agility test were altered during
the test during the fourth week of Ramadan compared to before Ramadan. These
authors mentioned that the decrease observed was not related to fasting but
rather to environmental and motivational factors since the tests performed are
not of sufficient duration to be influenced by the availability or not of the
energy substrates. Thus the tests are unlikely to be affected by the low
caloric intake associated with the month of Ramadan. In addition,
Kirkendall et al., (2008) reported performance in sprint (7 x
30 m), dribbling (McGregor et al., 2002), CMJ, pass testing
(Ali et al., 2007) and agility 4-line agility
test(Rösch et al., 2000) were not affected in footballers
continuing their training during Ramadan. The authors explained the stability
or sometimes the improvement in performance observed during the month of
fasting by the effect of maintaining the same intensity and duration of the
training sessions and by the fact that the players live together during the
period of investigation. Similarly, Chaouachi et al. (2009)
did not report a change in performance in sprint (5 m, 10 m, 30 m),
squat jump and CMJ recorded at high level judokas who maintained a high
intensity and high training volume during Ramadan.
However, the average power recorded during the 30-second
repetitive jump test decreased towards the end of Ramadan. Chaouachi et
al., (2009) explain this decline by the effects of reduced
carbohydrate consumption and lower body mass, which results in decreased
buffering capacity during intense muscle contractions. On the other hand,
Girard and Farooq (2011) studied the impact of fasting during
the month of Ramadan on the ability to repeat sprints in children aged between
10 and 14 years and they showed that the performance during repeated
20
sprints had deteriorated at the end of Ramadan and this effect
persisted for at least two weeks while the fatigue resistance was preserved.
II - The heart rate variability
This part is devoted to the heart rate variability (H.R.V).
We will successively present the following points:
· The branches of the autonomic nervous system that
innervate the heart and influence its rhythm and contractions.
· Study of the cardiac variability on the two analysis
plans (temporal and frequency) and the parameters measured.
· Physiological interpretation of different parameters of
heart rate variability II.1. Autonomic nervous system
The autonomic nervous system (ANS), also called vegetative
nervous system (VNS) or neurovegetative intervenes in the regulation of many
functions of the body. It can be considered as a common final pathway,
stretched between the neural axis and effectors organs, and subject to the
double influence of peripheral afference and supra segmental centres of the
central nervous system. Its involvement does not lead to paralysis but
dysfunction of the organ that innervates, which organ most often has a specific
functional autonomy that the vegetative system adapts incessantly to the
conditions of the environment (Mathias &Bannister,
2002).
The ANS thus has a role of modulator and regulator of the
unconscious vegetative life while fine-tuning the activities of the organs,
with respect to the environment and respecting their independence
(Appenzeller & Oribe, 1997, Mathias, 2000). It acts on
metabolism and electrolyte balances, blood pressure, body temperature, blood
composition and is involved in the functioning of the cardiovascular,
respiratory and digestive systems (Guyton, 2006).
ANS effectors are the tissues and organs responsible for
maintaining homeostasis, mainly the myocardium, the smooth muscles of the
vessels and hollow viscera, such as the bronchi, the digestive tract and the
bladder, as well as the glands and secretory cells. Its functioning is reflex,
unconscious and autonomous (Spalding, 1969) but is under
control of other parts of the nervous system.
21
ANS reactions are fast, of the order of a second, and are
distributed in the body, whereas the somatic nervous system has reactions of
the order of a millisecond and are local. Moreover, the ANS can also solicit
the somatic nervous system to feel sensations, such as thirst, hunger, urge to
urinate, or pain. The involvement of the ANS means a dysfunction of the organ
and not a stop. The organs have functional autonomy that the SNV only adapts.
If it is no longer active, the organs continue to function but their activities
are no longer maintained in homeostasis and in the reaction to aggression
(Langley, 1921, Cannon, 1929). While an attack of the somatic
nervous system will cause a loss of function, identical to anaesthesia or
paralysis.
The ANS is composed of two subsystems: sympathetic and
parasympathetic. At the level of an effectors, there is a double innervations
by the two sub-systems whose effects are conjugated, opposed or succeed one
another. However, sweat glands, piloerector muscles, and some subcutaneous
vessels do not exhibit parasympathetic innervations. These two subsystems are
composed of afferents, specific centres located in the central nervous system
and an efferent pathway, formed by two neurons within the SNA. There are also
relays in the ANS outside the central nervous system, in cell clusters called
ganglia, between centres and effectors. We then distinguish Pre-ganglion
neurons, which have cell bodies located in the central nervous system (spinal
cord), and postganglionic neurons, so-called effectors, located in the ganglia
(Pruvost, 2007).
Many organs, such as the heart, have a double innervations;
sympathetic and parasympathetic. Now, the effects of the two branches of the
autonomic nervous system are antagonistic. Their actions interact constantly:
the parasympathetic influence is restricted by sympathetic influence and vice
versa. Nerve modulation on the heart causes a change in heart rate, called a
chronotropic effect. It should also be noted that the heart rate is also
influenced by hormonal control mediated through the bloodstream, but hormonal
control is less rapid and less powerful than direct nerve control
(Pocock & Richards, 2004). It has been suggested that
abnormal regulation of the autonomic nervous system is a biological process
leading to arrhythmias and cardiovascular events during stress
(Bhattacharyya & Steptoe, 2007). For example, an increase
in cardiovascular events has already been reported following earthquakes and
major sports competitions (Wilbert-Lampen et al., 2008).
It is therefore clear that the autonomic nervous system can
be divided into two major parts: the sympathetic nervous system and the
parasympathetic nervous system. Their origins are
22
found at different levels of the spinal cord and at the base
of the brain. The effects of these two systems are often antagonistic, but they
always work together, although for more methodological reasons we have to study
them separately.
II.1.1. The sympathetic nervous system
The sympathetic nervous system or orthosympathetic nervous
system is one of the three parts of the efferent autonomic nervous system. The
other two parts are the enteric nervous system and the parasympathetic nervous
system. The sympathetic nervous system is our system of action and struggle. It
prepares the body for stress situations. It is responsible for controlling a
large number of unconscious activities of the body, such as heart rate or
contraction of smooth muscles. It exerts its effects on target cells and organs
mainly via neurotransmitters called catecholamines (noradrenaline and, to a
lesser extent, adrenaline). Yet the sympathetic nervous system is not quite
superimposed on the adrenergic nervous system, its action sometimes passing
(some vessels, sweat glands) by a secretion of acetylcholine. For this, it
produces a massive discharge throughout the body and prepares it for action. A
violent and unexpected noise, a situation of fear or the last few seconds
before the start of a sports competition are all examples of the moment when
this massive discharge takes place. The effects of sympathetic stimulation are
important for the athlete.
· Increased heart rate and contraction force of the
heart,
· Dilatation of the coronary vessels and therefore
increased cardiac output,
· Muscle vasodilatation to bring more blood to the active
muscles,
· Vasoconstriction in other areas, diverting the blood mass
to the active muscles,
· Increased blood pressure, which improves muscle perfusion
and venous return,
· Increased metabolic level in response to increased
needs,
· Stimulation of mental activity that improves perception
and concentration,
· Liver release of glucose into the blood,
· Finally, functions that are not directly involved in
exercise function at a slower rate (renal function, digestion), which saves the
energy needed for movement.
These changes in the basal body function facilitate the motor
response. This highlights the importance of the autonomic nervous system to
acute stress or physical exercise (Wilmore & Costill,
1998).
23
II.1.2. The parasympathetic nervous system
The parasympathetic nervous system or vagal system is our
defense system. It is one of three divisions of the autonomic or visceral
nervous system, with the orthosympathetic nervous system and the enteric
nervous system. The nerve fibers of the parasympathetic system originate in the
cranial (nerve III, VII, IX, and X) and sacral parts of the spinal cord. It
controls the involuntary activities of the organs, glands, and blood vessels
together with one of the other parts of the autonomic nervous system: the
sympathetic nervous system (orthosympathetic).
The parasympathetic influence is modulated by the release of
acetylcholine, the latter is responsible for the slowing of the heart rate
(cardio-moderator). This acetylcholine plays a major role in the digestive and
urinary functions; this secretion is more active when one is calm or at rest.
Its effects are generally opposed to those of the sympathetic system and leads
to:
· a drop in the heart rate,
· an increase in gastric, salivary and intestinal
secretions.
· a loosening of most sphincters of the gastrointestinal
tract.
II.2. Influence of autonomic nervous system on heart
rate
Although the heart has a specific functional autonomy, the
autonomic nervous system constantly adapts its frequency and contraction force
to different environmental conditions and influences. The parasympathetic
nervous system (via the vagus nerve or X) has a general effect on the heart
rate. The sympathetic nervous system generally increases cardiac activity.
Although these two systems interact continuously, the permanent parasympathetic
influence (vagal tone) is often the most intense, making the heart rate largely
dependent on vagal stimulation / inhibition.
II.3. Cardiac variability study
Blood pressure and heart rate fluctuate continuously and are
under the control of several regulatory systems: short-term regulation
represented by the central nervous system, baroreflex and choreflex systems;
medium-term regulation thanks to the hormonal systems (renin-angiotensin
system, vasopressin, natriuretic atrial factor ...), the tension-relaxation
phenomenon and the transfer of interstitial fluid to the plasma sector and vice
versa; and finally, a long-term regulation supported especially by the kidneys.
Blood pressure and heart
24
rate are therefore not constant phenomena: they vary
constantly. This variability can be defined as the set of variations of these
parameters around an average reference value and can be broken down into two
time scales:
· Variability over a 24-hour period, still termed circadian
or long-term.
· Variability over a period of a few minutes (usually 5
minutes), termed short-term variability, including spontaneous and unannounced
variations (effort, emotion, positional change ...).
Because of its ability to rapidly modulate blood pressure and
heart rate levels through the baroreflex system primarily (short-term
regulation), the activity of the autonomic nervous system can be studied by
measuring the variability of these two parameters. Over the last twenty years,
heart rate variability has become a non-invasive marker of autonomic nervous
system activity (Jourdan, 2008). The study of cardiac
variability is done on two different temporal and frequency planes
(Neto et al, 2005).
II.3.1. In the time domain
Time domain analysis is a simpler analysis than spectral
analysis. Measurements in the time domain are produced from arithmetic
calculations. There are two classes: on the one hand, the measurements derived
directly from the normal-to-normal intervals between two beats and, on the
other hand, the measurements derived from the differences of the
normal-to-normal intervals themselves, among the parameters which can be
measured by the analysis in the field of time:
· NN 50: number of successive RR intervals
greater than 50 ms.
· PNN50: NN50 divided by the total
number of intervals that expresses the high frequency variability mainly of
modulated parasympathetic origin.
· RMSSD: square root of the squared
differences of the successive RR intervals (the squared root of the mean of the
sum of the squares of differences between adjacent NN intervals) which also
expresses the high frequency variability mainly of parasympathetic origin,
modulated by the breathing. This measurement is preferable to pNN50 and
NN50.
· SDNN: (standard deviation of the RR
interval over the entire recording period, standard deviation of all NN
intervals) which gives information on the overall variability.
25
These indices are therefore a non-invasive method for studying
the cardiac response to stimulation of the autonomic nervous system. They
constitute a global approach to the influence of the autonomic nervous system.
However, some methodological precautions should be emphasized. Many of these
clues depend on the length of the recording. It is therefore necessary to
standardize this length in order to be able to compare these different
parameters. Consequently, it is imperative to only compare these parameters for
an identical recording length (Jourdan.G, 2008).
II.3.2. In the frequency domain
In recent years, the spectral analysis of cardiac
variability, based on the analysis of variations of RR intervals, has become
the reference tool for the study of the dynamic interactions between
parasympathetic and sympathetic controls (Malliani et al. 1991).
Spectral analysis then breaks down a complex signal like heart rate
into its constituents of frequency and quantifies the relative power of these
components (Jourdan.G, 2008).After mathematical processing, a
periodic signal of any shape (such as the heart rate, for example) appears in
fact as the superposition of a sum of sinusoids or elementary oscillations. The
fast Fourier transform allows the mathematical decomposition of a complex
record into its constituent or elementary elements without loss of information.
Each elementary sinusoid is mathematically defined by its amplitude and
frequency. The set of sinusoids then constitutes the spectrum. The resulting
graph shows on the abscissa, a frequency scale (in hertz, Hz) and on the
y-axis, an amplitude scale. It allows the study of different oscillations of
specific frequencies. In humans, the spectrum of the heart rate ranges from 0
to 0.4 Hz and can be divided into 3 areas of interest (on a recording of short
duration, 2 to 5 minutes) or in 4 areas of interest (on a long-term recording,
24 hours) (Anonymous, 1996).
The parameters that can be calculated from the spectral
analysis:
· Total power (ms2): Normal-to-normal interval
variance of the entire record.
· Ultrafast frequencies (ULF): from 0.0001 to 0.003 Hz
(only if 24 hours recording).
· Very low frequencies (VLF): from 0.003 to 0.04 Hz.
· Low Frequencies (LF): 0.04 to 0.15 Hz Oscillation in
this frequency band is known as Traube-Hering waves.
· High frequencies (HF): 0.15 to 0.4 Hz. The oscillation
in this frequency band is known as the Mayer wave.
·
26
VLF (ms2): Power in very low frequencies.
· LF (ms2): Power in the low frequencies.
· HF (ms2): Power in high frequencies.
LF and HF can also be in so-called normalized values, which
corresponds to the power of the frequency band considered divided by the total
power of the spectrum less VLF:
· HF (normalized): HF nu = 100 X HF / (HF + LF).
· LF (normalized): LF nu = 100 X LF / (HF + LF). The
values thus standardized and the LF / HF ratio then make it possible to
quantify, albeit in a simplified way, the sympathetic and vagal contribution to
the variability of the heart rate (Neto et al., 2005).
II.3.3. Relationship between spectral and temporal study
parameters
Neto et al., (2005) showed that in the
analysis of cardiac variability, many temporal and frequency domain variables
appear strongly correlated. These correlations are in fact a reflection of
their mathematical as well as physiological significance and interdependence.
Unless performing other analyzes than those commonly used in the frequency
domain and which are mentioned above, the variables conventionally used in the
frequency domain are therefore equivalent to those of the time domain.
Table I: Indices of human heart
rate variability in the frequency and time domains and their approximate
matches of 24-hour recordings. (Neto et al.)
Time variable Frequency domain Temporal domain Correspondence
PT Whole frequency scale but approximately <0.4
SDNN
ULF(VLT) 0,0001 à 0,0003 Hz SDNN,
SDANN
VLF(VLT) 0,0001 à 0,0003 Hz SDNN Index
LF(VCT) 0,0001 à 0,0003 Hz SDNN Index
HF(VCT) 0,0001 à 0,0003 Hz RMSSD,
PNN50
PT: total power of the spectrum; ULF: ultra low frequencies;
VLF: very low frequencies; LF: low frequencies; HF: high frequencies; SDNN:
standard deviation of the RR interval over the entire recording period; SDANN:
standard deviation of the mean RR intervals over 5-minute periods over the
entire recording period; SDNN index: mean of standard deviations of the RR
interval over 5-minute periods over the entire recording period; RMSSD: square
root of
27
squared differences of successive RR intervals; pNN50: this is
the NN50 divided by the total number of RR intervals.
II.3.4. Physiological interpretation of different
parameters of heart rate variability
The physiological basis for analyzing the short-term
variability of heart rate is based on different mechanisms of action and
control between the parasympathetic and the orthosympathetic systems.
Parasympathetic influences exert a rapid and dynamic control via the release of
acetylcholine and its action on the Muscarinic receptors are mainly reflected
by the high frequency component of heart rate variability.
Moreover, the orthosympathetic system is reflected by the
release of noradrenaline and its action on â-adrenergic receptors, which
exerts a slower influence and is manifested in the low frequency component of
the variability of the heart rate.
The short-term variability of heart rate is therefore an
indirect measure of autonomic nervous system activity. It is a reflection of
autonomous influences on the sinoatrial node, more than on the ventricular
myocardium. The analysis of heart rate variability, however, provides an
insight into the variations in autonomic tone associated with various
conditions. Two major components are studied: low frequencies (LF, from 0.04 to
0.15 Hz) and high frequencies (HF, from 0.15 to 0.4 Hz, synchronized with the
respiratory rhythm). While the high frequency band is clearly attributed to
vagal mechanisms (Akselrod et al., 1981, Malliani et al., 1991, Camm,
1996), several hypotheses have been advanced regarding the low
frequency band. The interpretation of this LF component is considered by some
to be a sympathetic modulation index (Rimoldi et al., 1990, Malliani
1991, Kamath & Fallen, 1993, Montano et al., 1994) and for others
as a parameter. Including both sympathetic and parasympathetic influences
(Akselrod et al., 1981, Appel et al., 1989).
Therefore, the low frequency-high frequency ratio can be
considered as a mirror of the sympathovagal balance or the influence of the
sympathetic system on the heart rate. A circadian rhythm of the sympathovagal
balance has been observed in the population: the LF spectral component
predominates during the day whereas it is the HF spectral component that is
predominant at night. There is therefore a marked decrease in the LF / HF ratio
between day and night. This observation reflects the day / night variation in
the influences of para- and
28
orthosympathetic systems (sympathetic predominance during the
day and vagal at night) (Furlan et al., 1991, Malliani et al.,
1991).
Part II : Material and
Methodes
I. 30
Participants
Nine male athletes playing football for at least 5 years in a
"professional league II" club aged 16.2 #177; 0.5 years and a size of 176 #177;
5 cm participated in our study after reading the different modalities of the
experimental protocol. The weight and height of the subjects were measured
using a scale and a height scale. The inclusion criteria consist of keeping
standard meal times (breakfast at 07:00: 00 #177; 1:00, lunch at 12:00: 00
#177; 1:00: 00 and dinner at 20:00: 00 #177; 1: 00) and sleep (sleep between
11:00 pm and 7:00 am + 1:00 am) before the start of the study. This criterion
allowed to provide a sample of participants having the same bedtime (23h: 00
#177; 00h: 30) and to raise (06h: 30 #177; 00h: 30). The subjects are
non-smokers and do not consume caffeine or alcoholic beverages. The first day
of the month of Ramadan of the year 1432 of Hegira corresponds to August 1,
2011 while the last day corresponds to August 30, 2011. The time elapsing from
the beginning of dawn until sunset was from 05h: 24 to 19h: 27 at the beginning
of Ramadan and from 05h: 47 to 18h: 53 at the end of the Holy month. During
this month, participants consume their last meal around 1:00 am and since then
refrain from eating and drinking until sunset.
II. Experimental procedures
The experimental protocol spanned three periods: two weeks
before Ramadan (BR), the end of the second week of Ramadan (R2) and the end of
the fourth week of Ramadan (R4). Before the beginning of the protocol, a
familiarization session is performed in order to avoid the effects of learning
that could occur with the repetition of the test sessions (Pincivero et
al., 1997). During this familiarization session, subjects became aware
of the nature of the test and the constraints of the experiment. Instructions
regarding sleep, diet and physical activity have been provided to the subjects
concerned. During the experimental period, subjects were asked not to perform
intense sporting activities the day before and during the day they were
assessed. During the same experimental period, subjects were reminded that
ingestions of caffeine-based foods and beverages are out of the question, as
anything that could increase their awakening.
The test sessions were conducted in the biology laboratory of
Farhat Hachad Hospital in Sousse at the same time of the day (between 14:00 and
17:00) in order to maintain identical experimental conditions. The subjects are
asked to use the same sports shoes at each session.
31
II.1. The Wingate test
The Wingate test was performed on a Monark model 894E (Monark
AB, Varberg, Sweden) with pedals fitted with footrests. The athlete performs
maximum effort for 30 seconds against a braking force. For each subject the
load is determined according to body weight according to the Bar-Or
(1987) optimization table (87g / kg of body weight). During the test,
subjects were strongly encouraged to motivate them. The test then allows us to
record average power and peak power during exercise.

Photo 1: Achievement of the Wingate test II.2
Recording of heart variability
The work begins with a period of stabilization of the
autonomous system. The subject being completely isolated from his comrades, he
is asked to lie down for ten minutes while being awake (he is asked about not
falling asleep, not to talk, and not to change too much their breathing)
(Cassirame, 2007).
From this moment, the protocol starts, which consists of
recording the heart rate with a Polar S810 watch (in RR mode):
1.
32
Recumbent position: in the supine position for ten minutes
without the subject making any movement,
2. Standing position: Follows a second period of ten minutes
during which the subject moves to the standing position,
3. During the effort: during the third period, the subject is
asked to pass on the cyclo-ergometer where he will pedal for 2'30 " at moderate
intensity as a warm-up, then the subject will execute the Wingate test (30 sec
of maximum effort) then continue pedalling for 2 min at empty load as active
recovery.

Photo 2 and 3: Recording heart variability at
rest
The environment is kept stable, with an average temperature
and atmospheric pressure of (25 #177; 1° C, 45%), a decrease in noise and
no movement around the person. The data was then analyzed using KUBIOS HRV 2.0
software for the study of cardiac variability.
The KUBIOS HRV 2.0. is a software that allows to study the
activity of the sympathovagal balance. Indeed, through this software, we can
analyze individually the sympathetic and parasympathetic effect on cardiac
function. Data obtained by POLAR S810 were transferred to KUBIOS, and analyzed
on time and frequency plans. This non-invasive method to study cardiac function
and its control by the vegetative nervous system has shown its credibility
which according to Gamelin et al. (2005), has achieved the
same results as a direct recording of the heart rate (ECG).
33
III. Statistical analysis
Statistical analysis of the data was performed using the
Statistica 6.01 software (Stat Soft, France). Values are expressed as mean
#177; standard deviation.
Data analysis was performed as follows:
· One-way analysis of variance (ANOVA) (Ramadan: BR, R2,
R4) for the Wingate test.
· One-way analysis of variance (ANOVA) (Ramadan: BR, R2,
R4) for cardiac variability parameters.
For each analysis, when the ANOVA shows a significant effect, a
Tukey post-hoc test is applied to compare the experimental data in pairs. All
observed differences are considered statistically significant for a probability
threshold of less than 0.05.
Part III : Results
I. 35
Anthropometric characteristics of the sample
Anthropometric parameters are shown in Table II. The analysis of
the variance did not show a significant effect for the weight and BMI
parameters that were not changed during the three measurement periods.
Table II: Mean #177; SD
anthropometric characteristics of the subjects during the second week, the
fourth week and before Ramadan (n = 9).
|
B R
|
R 1
|
R 2
|
|
|
|
|
Weight (KG)
|
65.78 #177; 5.71
|
65.55 #177; 5.56
|
64.74 #177; 6.11
|
|
|
|
|
|
|
|
|
BMI
|
21,23 #177; 1.73
|
21,16#177; 1.76
|
20.90 #177; 1.81
|
|
II. The Wingate test
The parameters calculated from the Wingate test during the second
week, the fourth week and before Ramadan are presented in Table 3.
Table III: Mean #177; SD of Wingate
test parameters during the second and fourth week of Ramadan and Before Ramadan
(n = 9).
|
B R
|
R1
|
R2
|
|
|
|
|
|
Ppic (W)
|
623,11#177;75,96
|
622,58#177;62,02
|
622,44#177;69,01
|
|
|
|
|
|
Ppic (W/kg)
|
9,51#177;0,58
|
9,48#177;0,63
|
9,62#177;0,59
|
|
|
|
|
|
Pave (W)
|
495,43#177;49,6
|
494,75#177;64,3
|
492,45#177;71,91
|
|
|
|
|
|
Pave (W/kg)
|
7,57#177;0,62
|
7,55#177;0,66
|
7,61#177;0,36
|
II.1. Average power (P ave)
The analysis of the variance does not show any significant
effect of the fast of Ramadan on the average power expressed in Watts.
Similarly, the relative average powers (W / Kg) relative to weight do not show
any significant difference between the three periods even though the values
have slightly decreased during these periods.
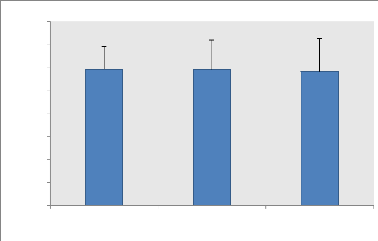
P ave (Watt)
450
400
600
550
500
350
300
250
200
BR
A R M R
R2 R3
F R
36
Figure 1: Average (#177; SD) of mean powers (W) of the Wingate
test recorded during the
second, fourth week and before Ramadan (n = 9).
II.2 Peak power (Ppic)
Variance analysis does not show a significant effect of
Ramadan fasting on Peak power expressed in Watts. Likewise, the relative peak
powers (W / Kg) show no significant difference between the different
measurement periods (BR, R1 and R2).
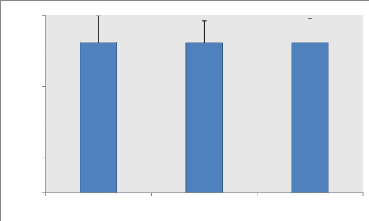
P.pic (Watt)
400
700
600
500
300
200
A R M R
R2 R3
F R
BR
Figure 2: Average (#177; SD) of the Peak (W) Wingate ratings
recorded during the second, fourth
week and before Ramadan (n = 9).
37
III. Effect of fasting Ramadan on the autonomic nervous
system
The parameters calculated from the cardiac variability analysis
during the second week, the fourth week and before Ramadan are presented in the
following tables:
Table IV: Mean (#177; SD) of the
analysis parameters of cardiac variability analysis in supine position
B R R 1 R 2
|
R R
|
1001 #177; 105
|
1054 #177; 83*
|
1081 #177; 89*
|
|
RMSSD
|
72, 88 #177; 21, 86
|
81, 91 #177; 14, 58*
|
90, 16 #177; 15, 55*
|
|
PNN50
|
39, 42 #177; 17, 18
|
45, 67 #177; 9, 62*
|
47, 25 #177; 11, 26*
|
|
L F (ms2)
|
324 #177; 126
|
304 #177; 101*
|
298 #177; 112*
|
|
H F (ms2)
|
1011 #177; 572
|
1082#177;659*
|
1181 #177; 623*
|
|
L F (nu)
|
38, 77 #177; 24, 12
|
31, 56 #177; 22, 3*
|
29, 44 #177; 20, 4*
|
|
H F (nu)
|
57, 39 #177; 20, 4
|
69, 33 #177; 32, 3*
|
75, 66 #177; 29, 12*
|
|
LF/HF
|
1, 69 #177; 0, 90
|
1, 37 #177; 0, 69 *
|
1, 24 #177; 0, 70*
|
RR, Time intervals between two successive RR peaks; RMSSD,
square root of squared differences of successive RR intervals: PNN50 percentage
of differences in successive RR intervals greater than 50 ms; LF, Power in the
low frequencies; HF, power in high frequencies; LF / HF, sympathovagal balance,
recorded during the second, fourth week and before Ramadan (n = 9). ** (p
<0.01), * (p <0.05): Significant difference from before Ramadan.
££ (p <0.01), £ (p <0.05): Significant difference between
the second and fourth week of Ramadan.
Table V: Mean (#177; SD) of the
analysis parameters of cardiac variability in standing position.
|
B R
|
R 1
|
R 2
|
|
R R
|
822 #177;93
|
872#177;77 a
|
883#177;82 a
|
|
RMSSD
|
31,93#177;13,96
|
39,37 #177;7,43 a
|
41,94#177;9,38 a
|
|
PNN50
|
7,01#177;5,15
|
12,75 #177;3,24 a
|
14,52#177;4,80 a
|
|
L F (ms2)
|
376 #177; 111
|
333 #177;93 a
|
321 #177;88 a
|
|
H F (ms2)
|
239#177; 144
|
276 #177;155 a
|
298#177;84 a
|
|
L F (nu)
|
69,33#177; 21,2
|
62,3 #177;19,2 a
|
59,88 #177;17,7 a
|
|
H F (nu)
|
32,11#177; 21,2
|
44,12 #177;22,2 a
|
51,22 #177;21,7 a
|
|
LF/HF
|
2,93#177;0,93
|
2,65 #177;0,89 a
|
2,31#177;0,93 a
|
RR, Time intervals between two successive RR peaks; RMSSD,
square root of squared differences of successive RR intervals: PNN50 percentage
of differences in successive RR intervals greater than 50 ms; LF, Power in the
low frequencies; HF, power in high frequencies; LF / HF, sympathovagal balance,
recorded during the second, fourth week and before Ramadan (n = 9). aa (p
<0.01), a (p <0.05): Significant difference from before Ramadan;
££ (p <0.01), £ (p <0.05): Significant difference between
the second and fourth week of Ramadan.
38
Table VI: Mean (#177; SD) of the
analysis parameters of the cardiac variability during the effort.
B R R 1 R 2
|
R R
|
491 #177; 99
|
521 #177; 71 b
|
533 #177; 68 b
|
|
RMSSD
|
9,77 #177; 3,96
|
16,57 #177; 2,91 bb
|
18,21 #177; 2,92 bb
|
|
PNN50
|
0 #177; 0
|
0 #177; 0,1
|
0 #177; 0
|
|
LF (ms2)
|
25 #177; 18
|
19 #177; 9 b
|
17 #177; 13 b
|
|
HF (ms2)
|
7 #177; 2
|
9 #177; 3
|
9 #177; 2
|
|
L F (nu)
|
79,33 #177; 12,33
|
69,22 #177; 12,44 b
|
66,33 #177; 13,55 b
|
|
H F (nu)
|
21,33 #177; 12,33
|
28,64 #177; 12,44 b
|
31,42 #177; 13,55 b
|
|
LF/HF
|
5,59 #177; 3,95
|
5,01#177; 3,81 b
|
4,91 #177; 2,79 b
|
RR, Time interval between two successive RR peaks; RMSSD,
square root of squared differences of successive RR intervals: PNN50 percentage
of differences in successive RR intervals greater than 50 ms; LF, Power in the
low frequencies; HF, power in high frequencies; LF / HF, sympathovagal balance;
recorded during the second, fourth week and before Ramadan (n = 9). bb (p
<0.01), b (p <0.05): Significant difference from before Ramadan;
££ (p <0.01), £ (p <0.05): Significant difference between
the second and fourth week of the month of Ramadan
39
III.1. Effect of Ramadan fasting on the sympathetic
system III.1.1. LF (ms2)

LF (ms2)
400
600 Supine Standing Effort
P.cou P.debou P.l'effo
500
300
200
100
0
*
a
b
*
a
b
Figure 3: Mean (#177; SD) LF (ms2)
values recorded during the second, fourth week and before Ramadan (n = 9).
* (p <0.05) Significant difference from before Ramadan
(supine position);
a (p <0.05) Significant difference from before Ramadan
(standing position); b (p <0.05) Significant difference from before Ramadan
(During effort).
· Supine position: the analysis of the variance
shows a significant effect F (2) = 12.67; p <0.05 Ramadan fasting on the
mean values LF (ms2) (Figure 3).
The post hoc analysis shows that the LF values recorded
before Ramadan are significantly higher than those measured in the middle and
at the end of the month of fasting P <0.05.
· Standing position: The analysis of the variance
shows a significant effect F (2) = 12.22, p <0.05 Ramadan fasting on the
mean values LF (ms2) (Figure 3).
The post hoc analysis shows that the LF values recorded
before Ramadan, when standing, are significantly higher than those measured in
the middle and at the end of the month of fasting p <0.05.
· 40
During effort: The analysis of the variance shows a
significant effect F (2) = 11,52; p <0.05 Ramadan fasting on mean values LF
(ms2) after exercise (Figure 3).
The post hoc analysis shows that the LF values recorded before
Ramadan, during exercise, are significantly higher than those measured in the
middle and at the end of the month of fasting (P <0.05).
III.1.2. LF (nu)
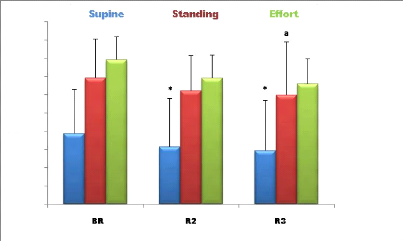
LF (nu)
100
40
90
80
70
60
50
30
20
10
0
a b
b
Figure 4: Mean (#177; SD) LF (nu) values
recorded during the second, fourth week and before Ramadan (n = 9).
* (p <0.05) Significant difference compared to before Ramadan
(supine position); a (p <0.05) Significant difference compared to before
Ramadan (standing position); b (p <0.05) Significant difference compared to
before Ramadan (During effort).
· Supine position: The Analysis of the variance
shows a significant effect F (2) = 11.66; P <0.05 Ramadan fasting on mean
values LF (nu) (Figure 4).
The post hoc analysis shows that the LF values recorded
before Ramadan, in supine position are significantly higher than those measured
in the middle and at the end of Ramadan (P <0.05).
· Standing position: The analysis of the variance
shows a significant effect F (2) = 11,23; P <0.05 of Ramadan fasting on the
mean LF values (Figure 4).
41
The post hoc analysis shows that the LF values recorded before
Ramadan, while standing, are significantly higher than those measured in the
middle and at the end of the month of Ramadan (P <0.05).
· During effort: The analysis of the variance
shows a significant effect F (2) = 10.55; P <0.05 of Ramadan fasting on mean
values LF (nu) (Figure 4).
The post hoc analysis shows that the LF values recorded
before Ramadan, during the effort, are significantly higher than those measured
in the middle and at the end of the month of Ramadan (P <0.05).
III.2. Effect of Ramadan fasting on the
parasympathetic system III.2.1. RMSSD (ms)

RMSSD (ms)
120
100
40
80
60
20
0
*
a
bb
a
bb
Figure 5: Mean (#177; SD) RMSSD(ms) values
recorded during the second, fourth week and before Ramadan (n =
9).
* (p <0.05) Significant difference compared to before Ramadan
(supine position);
a (p <0.05) Significant difference compared to before Ramadan
(standing position);
bb (p <0.05) Significant difference compared to before
Ramadan (During effort).
· Supine position: the Analysis of the variance
shows a significant effect F(2) = 11,52; P <0.05 Ramadan fasting on mean
values RMSSD (ms) (Figure 5).
42
The post hoc analysis shows that RMSSD values recorded before
Ramadan are significantly lower than those measured in the middle and end of
Ramadan (p <0.05).
· Standing position :The analysis of the variance
shows a significant effect F (2) = 11.88;(p <0.05) of Ramadan fasting on
mean values RMSSD recorded(ms) (Figure 5).
Post hoc analysis shows that standing RMSSD values before
Ramadan are significantly lower than those measured in the middle and at the
end of Ramadan (p<0, 05).
· During effort: The analysis of the variance
shows a significant effect F (2) = 16,62; (p <0.01) of Ramadan fasting on
mean RMSSD values recorded during effort (Figure 5).
The post hoc analysis shows that the RMSSD values recorded
during the effort, before Ramadan, are significantly lower than those measured
in the middle and at the end of the month of Ramadan (p <0.01).
III.2.2. PNN50 (%)

70
*
60
50
PNN50 (%)
40
30
a
20
10
0
*
a
Figure 6: Mean (#177; SD) of PNN50 (%)
recorded during the second, fourth week and before Ramadan (n
= 9).
* (p
<0.05) Significant difference compared to before Ramadan (supine
position);
a (p <0.05) Significant difference compared to before Ramadan
(standing position).
· Supine position: The analysis of the variance
shows a significant effect F (2) = 10.89 (p <0.05) of Ramadan fasting on
mean values PNN50, expressed in% (Figure 6).
43
Post hoc analysis shows that PNN50 percentages in supine position
before Ramadan are significantly lower than those measured in the middle and
end of Ramadan (p <0.05).
· Standing position: The analysis of the variance
shows a significant effect F (2) = 9.84 (p <0.05) of Ramadan fasting on the
values PNN50, expressed in% (Figure 6).
The post hoc analysis shows that the PNN50 percentages recorded,
while standing, before Ramadan are significantly lower than those measured in
the middle and at the end of the month of Ramadan (p <0.05).
· During effort: the analysis of the variance does
not show a significant effect of of Ramadan fasting on the PNN50 expressed in%
(Figure 6).
III.2.3. HF (ms2)

Supine Standing Effort
10
1600
1400
1200
1000
800
600
HF (ms2)
a
a
400
200
0
Figure 7: Mean (#177; SD) of the HF (ms2) values
recorded during the second, fourth week and before
Ramadan (n = 9).
* (p <0.05) Significant difference compared to before
Ramadan (supine position);
a (p <0.05) Significant difference compared to
before Ramadan (standing position).
· Supine position: The analysis of variance shows a
significant effect F (2) = 9.67 (p <0.05) of Ramadan fasting on mean HF
(ms2) values (Figure 7).
44
The post hoc analysis shows that the HF (ms2)
values recorded before Ramadan are significantly lower than those measured in
the middle and at the end of the month of Ramadan p <0.05.
· Standing position: Variance analysis shows a
significant effect F (2) = 10.22; (p <0.05) of Ramadan fasting on the
average values HF (ms2) (Figure 7).
The post hoc analysis shows that the HF values recorded
before Ramadan, while standing, are significantly higher than those measured in
the middle and at the end of the month of Ramadan P <0.05.
· During the effort: Variance analysis does not
show a significant effect of Ramadan fasting on mean LF values during exercise
(Figure 7).
III.2.4. HF (nu)
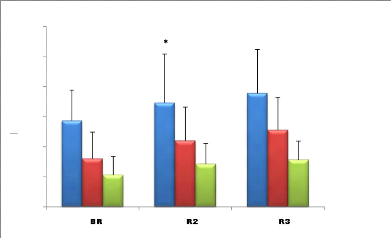
HF (nu)
120
100
40
80
60
20
0
Supine Standing Effort
a
b
*
a
b
Figure 8: Mean (#177; SD) of the HF (nu) values
recorded during the second, fourth week and before Ramadan (n =
9).
* (p <0.05) Significant difference compared to before Ramadan
(supine position);
a (p <0.05) Significant difference compared to before Ramadan
(standing position);
b (p <0.05) Significant difference compared to before Ramadan
(During effort).
· Supine position: The analysis of the variance
shows a significant effect F (2) = 9.67; (p <0.05) of Ramadan fasting on
mean HF (nu) values (Figure 8).
Post hoc analysis shows that HF values recorded before Ramadan
are significantly lower than those measured in the middle and at the end of
Ramadan p <0.05.
·
45
Standing position: The analysis of variance shows a
significant effect F (2) = 10.29 (p <0.05) of Ramadan fasting on mean HF
(nu) values (Figure 8).
Post hoc analysis shows that the HF values recorded before
Ramadan, while standing, are significantly lower than those measured in the
middle and at the end of the month of Ramadan p <0.05.
· During effort: The analysis of the variance
shows a significant effect F (2) = 12.52 (p <0.05) of Ramadan fasting on
mean values HF (nu) (Figure 8).
Post hoc analysis shows that HF values recorded prior to
Ramadan during exercise are significantly lower than those measured in the
middle and end of Ramadan (p <0.05).
III.3. Effect of Ramadan fasting on sympathovagal
balance
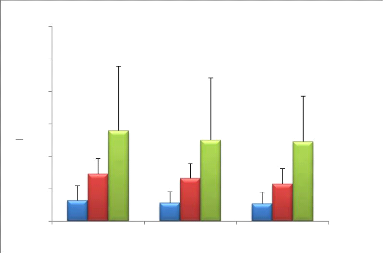
6
LF / HF
4
Supine Standing Effort
b
8
*
a
A R BR R1 R2
M
2
0
b
12
10
a
*
Figure 9: Mean (#177; SD) values (LF / HF)
recorded during the second, fourth week and before Ramadan (n = 9). * (p
<0.05) Significant difference compared to before Ramadan (supine position);
a (p <0.05) Significant difference compared to before Ramadan (standing
position); b (p <0.05) Significant difference compared to before Ramadan
(During effort).
· Supine position: Variance analysis shows a
significant F (2) = 9.82 (p <0.05) effect of Ramadan fasting on LF / HF
ratios (Figure 9).
The post hoc analysis shows that the LF / HF ratios, in supine
position, before Ramadan are significantly higher than those measured in the
middle of the month and at the end of the month of Ramadan (p <0.05).
·
46
Standing position: The analysis of the variance shows a
significant effect F (2) = 8.52; (p <0.05) of Ramadan fasting on the mean
values of the LF / HF ratios (Figure 9).
Post hoc analysis shows that LF / HF ratios before Ramadan,
in standing position, are significantly higher than those measured in the
middle and end of Ramadan (p <0.05).
· During effort: the analysis of the variance
shows a significant effect F (2) = 12,32; (p <0.05) of Ramadan fasting on LF
/ HF ratios (Figure 9).
Post hoc analysis shows that pre-Ramadan LF / HF ratios
during exercise are significantly higher than those measured in the middle and
end of Ramadan (p <0.05).
III.4. Effect of fasting Ramadan on the durations RR
(ms)

R R (ms)
1400
1200
1000
400
800
600
200
0
BR R1 R2
Supine Standing Effort
*
b
*
a
b
Figure 10: Mean (#177; SD) of the RRs (ms)
recorded during the second, fourth week and before Ramadan (n
= 9).
* (p <0.05) Significant difference compared to before
Ramadan (supine position);
a (p <0.05) Significant difference compared to before
Ramadan (standing position);
BR R1 R2
b (p <0.05) Significant difference compared to before Ramadan
(During effort).
47
Table VII: Heart rate averages
(bpm) recorded before, in the middle and at the end of the month of Ramadan (n
= 9).
|
B.R
|
R 1
|
R 2
|
F C (bpm) Supine
|
59,94
|
56,92
|
55,50
|
F C (bpm) Standing
|
72,99
|
68,80
|
67,95
|
F C (bpm) Effort
|
122,19
|
115,16
|
112,57
|
|
· Supine position: the analysis of variance shows
a significant effect F (2) = 13.2 (p <0.05) of Ramadan fasting on RR
durations (Figure 10).
Post hoc analysis shows that RR durations recorded before
Ramadan are significantly lower than those measured in the middle and at the
end of Ramadan (p <0.05).
· Standing position: Variance analysis shows a
significant effect F (2) = 8.22 (p <0.05) of Ramadan fasting on RR durations
(Figure 10).
The post hoc analysis shows that the RR durations recorded in
the standing position before Ramadan are significantly lower than those
measured in the middle and at the end of the month of Ramadan (p <0.05).
· During effort: the analysis of the variance
shows a significant effect F (2) = 17.6 (p <0.05) of Ramadan fasting on the
average RR durations (Figure 10).
The post hoc analysis shows that RR durations recorded during
the effort before Ramadan are significantly lower than those measured in the
middle and at the end of the month of Ramadan (p <0.05).
Part IV : Discussion
49
The objective of this study is to evaluate the effects of Ramadan
fasting on:
1. Performances during a Wingate test,
2. The modulation of sympathetic and parasympathetic system
activity on cardiovascular function in adolescents who have been playing
football for at least 5 years.
Our results showed that Ramadan fasting had no effect on
anthropometric parameters and performances during a Wingate test. Moreover, our
results revealed a modulation of the autonomic system by the increase of the
parasympathetic participation and by the decrease of the sympathetic effect.
I. The effects of fasting Ramadan on body
weight
The results of the present study found that the effect of
fasting on body weight does not show a significant difference during the month
of Ramadan compared to the control period (before Ramadan), this is confirmed
by other previous studies (El Ati et al., 1995, Finch et al., 1998,
Ramadan 2002, Souissi et al., 2007, Zerguini et al., 2007, Meckel et al., 2008,
Chennaoui et al., 2009, Sweileh et al. al., 1992). Moreover,
Sweileh et al., (1992) reported that dehydration exists only
during the first week of Ramadan as it returns to its pre-Ramadan values during
the fourth week of the month of fasting.
Ramadan et al., (1999) also noted a
significant increase in osmolarity among sedentarians, which is not the case
for athletes who continue to train during Ramadan.
Karli et al., (2007), confirmed the results
of Ramadan et al., (1999) in their study of international
athletes, where they conclude that the fluid balance has not changed
significantly. We can deduce the same conclusions since our subjects are
athletes who did not interrupt their sports activities during the holy
month.
II. The effects of Ramadan fasting on Wingate test
performances
Our results showed that the peak powers and the average powers
recorded during the Wingate test do not reveal a significant difference during
the second, fourth week of Ramadan compared to the pre-Ramadan control
session.
50
This confirms the results of some previous studies that looked
for the fasting effect of Ramadan on anaerobic performance especially for the
Wingate test. Indeed, Souissi et al., (2007a) showed that the
muscle powers recorded during the morning Wingate test are not affected during
the month of Ramadan. On the other hand, the muscular powers are diminished
during the fourth week compared to before Ramadan for the sessions realized in
the afternoon.
In the same vein, Karli et al., (2007) showed
that the average powers did not decrease significantly during Ramadan and that
the peak powers showed an increase in nine athletes who continued to train
normally during Ramadan.
On the other hand, other studies have led to other results.
Indeed, Chaouachi et al., (2009) showed that the power
recorded during the 30-sec repeated jump test decreased at the end of Ramadan
compared to before Ramadan. The study of Abedelmalek (2008)
showed that the average powers decreased significantly during the
second and fourth week of Ramadan compared to after Ramadan. The same study
showed that the Peak powers also declined significantly at the end of Ramadan
compared to after Ramadan. In this study, the author explained these decreases
in mean powers and peak powers by two essential factors: calorie restriction
and sleep deprivation; this is not the case of our study which showed that the
peak powers and the average powers were not affected by the fast of the month
of Ramadan.
Several factors can explain this stability of performances
during Wingate test found in our study. Indeed, the short duration of this test
(30s) and its anaerobic nature do not seem to be influenced by the availability
or not of the Energetic substrates. Thus, the test is slightly affected by the
reduced caloric intake relating to fasting Ramadan (Zerguini et al.,
2007).
In addition, our subjects did not stop their training during
Ramadan (2 to 4 training sessions per week). This confirms previous studies
(Rösch et al., 2000, McGregor et al., 2002, Ali et al., 2007,
Kirkendall et al., 2008) which explained the stability or even
sometimes the improvement in performance observed during the month of fasting
by the effect of maintaining the same intensity and duration of training
sessions.
With regard to partial sleep deprivation during Ramadan, data
available in the literature have shown that anaerobic performance seems to be
little affected by this factor (Mougin et al., 1996, Bambaeichi et al.,
2005, Reilly and Waterhouse, 2009). On the other hand,
51
Souissi et al., (2007b) have shown that the
effects of total and partial sleep deprivation are mainly related to the time
of testing. Indeed, these authors have shown that sleep deprivation has
negative effects only during the afternoon and evening, while the average
powers were only diminished at the end of Ramadan for sessions performed
between 10am and 11am. These results are discordant with ours, despite the fact
that we recorded a slight decrease that was not significant during the periods
of the test (between 15h and 17h).
In partial conclusion we say that, the performance stability
of the peak powers and the average powers during Wingate test during and out of
Ramadan can be explained by several factors:
1. the continuity of physical activity practice during
Ramadan,
2. the nature of the test itself and its short duration that
appears to be little affected by calorie deficit during the month of fasting or
sleep / wake rhythm disruption that results in decreased performance when
exercises require sensorimotor coordination or cognitive processes
(Mougin et al., 1996) which is not the case in our study
(purely physical test).
III. The effect of fasting Ramadan on the heart rate
variability
Ramadan fasting has a significant effect on RR duration (ms)
and sympathovagal balance at rest and during exercise. Indeed, under these
conditions, there is a lower heart rate (bpm) from one session to another
(Table VII); supine (59.94 Versus 56.92 Versus 55.50), standing (72.99 Versus
68.80 Versus 67.95) and during the effort interval (122.19 Versus 115.16 Versus
110,29) associated with an increase in parameter values representing
parasympathetic activity (HF« ms2», HF«nu»,
RMSSD, PNN50) (Tables IV, V, VI): (Akselrod et al., 1981; et al., 1991,
Camm, 1996) and the decrease in the values of the parameters
representing the sympathetic activity (HF« ms2»,
HF«nu») (Tables IV, V, VI) (Rimoldi et al., 1990; , 1991,
Kamath & Fallen, 1993, Montano et al., 1994), and consequently a
decrease in the LF / HF ratio; some authors have adapted this ratio as an
indicator of sympathetic activity (Yamamoto et al., 1991),
others as an index of sympathovagal balance (Pagani et al.,
1986).
This significant increase in RR (ms) duration, which reflects
a decrease in mean heart rate, is confirmed with the study by Hussain
et al., (1987); these authors found a significant decrease in heart
rate at rest throughout the month of fasting in male subjects. Similarly,
Karli et al.,
52
(2007) found a nonsignificant decrease heart
rate at rest during Ramadan compared to before Ramadan.
In addition, Zoladz et al., (2005) found a
decrease in heart rate of 10 beats / min during exercise at each workload that
starts at 30 W and ends at 150 W of fasting overnight. Similarly,
Ramadan et al., (1999) found a decrease in heart rate at
submaximal exercise during the month of Ramadan compared to the control
session.
Explanations of this phenomenon differ from one researcher to
another. Husain et al., (1987) explained this decrease in
heart rateat rest by the decrease in metabolic activity which is under the
influence of reduced sympathetic activity; these authors added that the
increase in religious devotion during Ramadan results from a solicitation of
the mental state which tends to lower metabolic rate and heart rate.
Zoladz et al., (2005) explained the
significant decrease in heart rate following a fasting night by increasing the
plasma level of noradrenaline leading to an increase in systemic vascular
resistance, and thus, the solicitation of arterial baroreceptors leading to
vagal stimulation (Schachinge et al., 2001 and Malpas, 2004 cited by
Zoladz et al., 2005). It is also known that diet can influence
cardiovascular regulation in healthy subjects at rest (Hoost et al.,
1996, Karpovich & Sinning, 1980). On the other hand, it has been
shown that the effect of diet (abstention or food intake) can influence the
cardiorespiratory activity by neuronal or hormonal pathways (Kearney et
al., 1996).
Other research has also shown that the secretion of leptin
(satiety hormone) and ghrelin (the hormone of hunger) can influence
cardiovascular activity (Haynes et al., 1987). According to
Matsumura, (2002, 2003) the injection of leptin activates the
sympathetic nervous system, and on the contrary, the injection of ghrelin
significantly decreases the heart rate in rabbits.
The decrease in resting and exercise heart rate can be
explained by increased secretion of ghrelin and a decrease in leptin secretion
during Ramadan, so that Zoladz et al.(2005) found no
significant difference between the secretion of these two fasting hormones
compared to the control session. According to Zoladz, this is probably due to
the short duration of fasting (one night) observed during this protocol.
Through the study of cardiac variability, our results
reflected a modulation of the autonomic nervous system by the decrease of the
parameters reflecting the sympathetic activity on the frequency plane (LF
« ms2 », LF « nu ») (FIG. 4)
and the increase of the parameters
53
reflecting the frequency-domain activity of the
parasympathetic system ("HF « ms2 », HF
« nu ») and their temporal correspondences (PNN50, RMSSD) (FIGS. 5,
6) (Neto et al., 2005) during the month of Ramadan.
We also found a significant decrease in the LF / HF ratio (FIG
9), which reflects sympathovagal activity or also the variation in sympathetic
activity, this decrease is explained by the decrease of the LF values (Tables
IV, V, VI) and the increase of the HF values (Tables IV, V, VI) during the holy
month.
These results may be a good explanation for the decrease in
heart rate during Ramadan and confirm the hypotheses of Husain et al.,
(1987). These authors explained the decrease in heart rate during the
holy month by a reduction in sympathetic tone.
These results may be consistent with the explanation given by
Zoladz et al., (2005) who attribute the decrease in heart rate
observed during exercise during abstinence by increasing systemic vascular
resistance and vagal stimulation via the baroreceptors.
We can also add the change in the daily habits of Muslims
during Ramadan, favoring sedentarism because Muslims tend to sleep late by
watching television, praying or reading (Afifi et al.,
1997).
In addition, general fatigue, reduced feeling of well-being,
and impaired cognitive function are the result of changes in eating habits and
sleep deprivation during Ramadan (Kadri et al., 2000; Leiper et al.
2003, Roky et al., 2004). It has also been shown that the month of
fasting is accompanied by a decrease in alertness, probably because of the
absence of lunch, which usually leads to falling asleep (El Kalifi,
1998).
The major changes in the rhythm of life in Ramadan, mainly
affecting food intake and sleep (Chaouachi et al., 2008, Maughan et
al., 2008a, Leiper et al., 2008) can also explain this increase in
tone. parasympathetic identified in our results. Indeed, fasting reduces the
basic metabolism in the absence of digestion. In addition, it has already been
shown that digestion accelerates the heart rate for 2 or 3 hours
(Karpovich, Sinning, 1980).
It is known that digestion activates the sympathetic
tone (Guyton, 2006) so fasting tilts the sympathovagal balance
towards parasympathetic tone which is confirmed by the study of
Al-Hazmi et al., (2009) who found a decrease in LF / HF ratio
during the holy month compared
54
to the control session according to Ramadan. They also
concluded that fasting protects the heart and minimizes the risk of heart
attacks.
Nerve modulation on the heart causes a change in heart rate,
called a chronotropic effect. It should also be noted that the heart rate is
also influenced by hormonal control mediated through the bloodstream, but
hormonal control is less rapid and less powerful than direct nerve control
(Pocock & Richards, 2004). This may explain the findings
found in some studies that show a decrease in heart rate despite the increase
in noradrenaline levels (Zoladz et al., 2005).
Indeed, fasting is a phenomenon of long duration; therefore
the decrease in heart rate may also be related to the decrease in the secretion
of the accelerating hormones of the heart rate during Ramadan; this mechanism
remains to be verified.
Our results show a modulation of the autonomic system by the
increase of the participation of the parasympathetic system (HF, HF nu, PNN50,
NN50) and the decrease of the sympathetic effect (SDNN, LF, LF nu) during the
month of fasting on the whole organism, and especially on the heart. The
explanation of this phenomenon can be attributed to two essential factors
related to the changes of life rhythm during Ramadan:
1. Sleep deprivation observed at night and,
2. The food intake.
There will consequently be a hormonal and nervous response to
these two major changes that are accentuated by the psychological factor
related to the specific spiritual environment, and the typical religious
climate created by the holy month of the Muslims.
Conclusion
56
In this study, we have tried to answer two main objectives:
· Recognize the effect of Ramadan fasting on anaerobic
sports performance through a laboratory test (the Wingate test).
· Identify the effect of Ramadan fasting on the activity
of the vegetative nervous system including the activity of sympathetic and
parasympathetic systems through a study of cardiac variability.
Our results showed that Ramadan fasting did not have any
effects:
1. On anthropometric parameters,
2. On performances during a Wingate test,
3. On the other hand, our results revealed a modulation of
the autonomous system by the increase of the participation of the
parasympathetic system and the decrease of the effect of the sympathetic
one.
The stability of performances during the Wingate test during
and out of Ramadan concerning essentially the peak powers and the average
powers can be explained by the continuity of physical activity practice during
Ramadan for our subjects. The nature of the test itself and its short duration
appear to be unaffected by caloric deficiency during the fasting month or by
sleep / wake disruption which results in decreased performance when exercises
require sensorimotor coordination or cognitive processes, which is not the case
in our study (purely physical test).
Our study showed that Ramadan fasting causes a decrease in
resting and exercise heart rate in response to vegetative nervous system
modulation through increased parasympathetic tone and decreased sympathetic
tone. This could be explained by the spiritual atmosphere created during the
holy month and the abstinence from eating which decreases the basic metabolism
in the absence of digestion.
Since this study is descriptive, we plan in the future to
continue our experiments in order to define more specifically the precise
mechanisms responsible for the modulation of the vegetative nervous system
during Ramadan with the different age groups (children, old people) and the
different training levels (high level, sedentary).
57
Bibliography
58
-A-
Abedelmalek S (2008).The effects of Ramadan
fasting on performance and interleukins during Wingate test, Master's thesis in
sports science.
Afifi Z.E (1997). Daily practices, study
performance and health during the Ramadan fast. J. R. Soc. Health
117:231-235.
Al Suwaidi J, Bener A, Gehani A.A, Behair S, Mohanadi
D.A, Salam A.A.L, Bin ali H.A (2006). Does the circadian pattern for
acute cardiac events presentation vary with fasting. J. Postgrad. Med.
52:30-33.
Ali A, Williams C, Hulse M, Strudwick A, Reddin J,
Howarth L, Eldred J, Hirst M, McGregor S (2007). Reliability and
validity of two tests of soccer skill. J.Sports Sci.25 (13) : 1461-70.
Al-Hazmi A, Hossam A and Zaher M.
(2009).Effect of Ramadan Fasting on Heart Rate Variability. Sci. Med. J.,
April; 21 (2): 41-49
Akselrod S., Gordon D , and. Ubel F. A
(1981). Power spectrum analysis of heart rate fluctuation: A
quantitative probe of beat-to-beat cardiovascular control. Science. 1981, 213
(4504), pp. 220-222.
Aloui A (2010). Effects of Ramadan fasting on
performances in repeated sprints and their diurnal fluctuations, Master's
thesis in sports sciences.
American College of Sports Medicine, Sawka M.N, Burke
L.M, Eichner E.R, Maughan R.J, Montain S.J, Stachenfeld N.S (2007).
American College of Sports Medicine positionstand. Exercise and fluid
replacement. Med. Sci. Sports Exerc. 39(2):377-90.
Angel J.F, Schwartz N.E (1975). Metabolic
changes resulting from decreased frequency in adult male Muslims during the
Ramadan fast. Nutr. Rep 11: 29-38
Anonymous (1996). Heart rate variability:
standards of measurement, physiological interpretation and clinical use. Task
Force of the European Society of Cardiology and the North merican Society of
Pacing and Electrophysiology. Circulation, 93(5):1043-65.
Appel M.L, Berger R.D, Saul J.P, Smith J.M., and Cohen
R.J (1989). Beat to beat variability in cardiovascular variables:
Noise or music? Journal of the American College of Cardiology. 1989, 14, pp.
1139-1148.
AppenzellerO and OribeE(1997).The Autonomic
Nervous System. An introduction to basic clinical concepts. Amsterdam: Elsevier
Medical Press, 1997.
Aragon-Vargas L.F(1993), Effects of fasting on
endurance exercise .Sport Med;16 : 255-65.
Armstrong L.E, Costill D.L, Fink W.J (1985).
Influence of diuretic-induced dehydration on competitive running performance.
Med. Sci. Sports Exerc. 17(4) : 456-61.
-B-
BaHammam A (2005). Assessment of sleep patterns,
daytime sleepiness, and chronotype during Ramadan in fasting and non fasting
individuals. Saudi. Med. J. 26(4) : 616-22.
BaHammam A, Alrajeh M, Albabtain M, Bahammam S, Sharif M
(2010). Circadian pattern of sleep, energy expenditure, and body
temperature of young healthy men during the intermittent fasting of Ramadan.
Appetite 54:426-429.
Bambaeichi E, Reilly T, Cable NT, Giacomoni M
(2005). Influence of time of day and partial sleep loss on muscle
strength in eumenorrheic females. Ergonomics 48 (11-14):1499- 511.
Bar-Or O (1987). The Wingate anaerobic test. An
update on methodology, reliability and validity. Sports Med. 4(6) : 381-94.
59
Bar S.I (1999). Effects of dehydration on
exercise performance. Can J Appl Physiol ; 24:164-172
Beltaifa L, Bouguerra R, Ben Salma C, Jabrane H,
El-Khadhi A, Ben Rayana M.C, Doghri T (2002). Food intake and
anthropometrical and biological parameters in adult Tunisians during fasting at
Ramadan. East. Mediterr. Health J. 8:603-611.
Bhattacharyya M.R and Steptoe A (2007). A.
Emotional Triggers of Acute Coronary Syndromes: Strength of Evidence,
Biological Processes, and Clinical Implications.Prog.Cardiovasc.Dis. 49
(5):353-65.
Bigard A.X, Boussif M, Chalabi H, Guezennec C.Y
(1998). Alterations in muscularperformance and orthostatic tolerance
during Ramadan. Aviat. Space Environ. Med. 69 (4) : 341-346.
Bogdan A, Bouchareb B, Touitou Y (2001).
Ramadan fasting alters endocrine and neuroendocrine circadian patterns.
Meal-time as a synchronizer in humans, Life Sci. 68 (14):1607-1615.
Bouhlel E, Salhi Z, Bouhlel H, Mdella S, Amamou A,
Zaouali M, Mercier J, Bigard X,Tabka Z, Zbidi A, Shephard R.J (2006).
Effect of Ramadan fasting on fuel oxidation during exercise in trained male
rugby players. Diabetes Metab. 32 (6) : 617-24.
Bouhlel E, Zaouali M, Miled A, Tabka Z, Bigard AX,
Shephard R (2008). Ramadan fasting and the GH/IGF-1 axis of trained
men during submaximal exercise. Ann. Nutr. Metab. 52:261-266.
Burke L.M, Loucks A.B, Broad N (2006). Energy
and carbohydrate for training and recovery. J. Sports Sci. 24(7) : 675-85.
-C-
Camm (1996) Task Force of the European
Society of Cardiology and the North American Societyof Pacing and
Electrophisiology. Heart rate variability. Standards of measurement,
physiologicalinterpretation, and clinical use. European Heart Journal. 1996,
17, pp. 354-381.
60
CannonW.B (1929). Bodily
Changes in Pain, Hunger, Fear and Rage. New York, Norton.
61
Cassirame J, Tordi N, Mourot L, Rakobowchuk M, Regnard
J (2007). The use of a new beat-to-beat heart rate recording system
for traditional heart rate variability analysis , Science & Sports (2007),
doi: 10.1016/j.scispo.2007.07.006.
Chaouachi A, Chamari K, Roky R, Wong P, Mbazaa A,
Bartagi Z, Amri M (2008). Lipid profiles of judo athletes during
Ramadan. Int. J. Sports Med. 29 (4) : 282-288.
Chaouachi A, Coutts A.J, Chamari K, Wong del P,
Chaouachi M, Chtara M, Roky R, Amri M (2009). Effect of Ramadan
intermittent fasting on aerobic and anaerobic performance and perception of
fatigue in male elite judo athletes. J. Strength Cond. Res. 23 (9) : 2702-9.
Chennaoui M, Desgorces F, Drogou C, Boudjemaa B,
Tomaszewski A, Depiesse F, Burnat P, Chalabi H, Gomez-Merino D (2009).
Effects of Ramadan fasting on physical performance and metabolic, hormonal, and
inflammatory parameters in middle-distancerunners. Appl. Physiol. Nutr. Metab.
34:587-594.
-D-
Danguir J, Nicolaidis S (1979). Dependence of
sleep on nutrients' availability. Physiol. Behav. 22(4):735-40.
Dijk D.J, Duffy J.F, Czeisler C.A (1992).
Circadian and sleep/wake dependent aspects of subjective alertness and
cognitive performance. J. Sleep Res. 1(2):112-7.
-E-
El Ati J, Beji C, Danguir J (1995). Increased
fat oxidation during Ramadan fasting in healthy women: an adaptative mechanism
for body weight maintenance. Am. J. Clin. Nutr. 62:302- 307.
El Kalifi K (1998). Les variations circadiennes
de la vigilance pendant le Ramadan. Thèse de la faculté de
médecine et de pharmacie de Casablanca.
62
-F-
Finch G.M, Day J.E, Razak,Welch D.A, Rogers P.J
(1998). Appetite changes under free living conditions during Ramadan
fasting. Appetite 2:159-170.
Frost .G, Pirani .S (1987). Meal frequency and
nutritional intake during Ramadan: a pilot study. Hum. Nutr. Appl. Nutr.
41:47-50.
Furlan R, GuzzettiS, CrivellaroW, DassiS, TinelliM,
Baselli, CeruttiS, LombardiF, PaganiM and Malliani A (1990).
Continuous 24-hour assessment of the neural regulation of systemic arterial
pressure and RR variabilities in ambulant subjects. Circulation,
81(2):537-47.
-G-
Gamelin F.X , Berthoin S , Bosquet L (2005)
Validity of the polar S810 Heart rate monitor to measure R-R Intervals at rest
, Medicine and science in sport and exercise. P: 887 - 893
Girard O, Farooq A (2011). Effects of Ramadan
fasting on repeated sprint ability in young children. Sci sports
doi:10.1016/j.scispo.2011.09.006
Guyton.A (2006). Precise book of medical
physiology.
-H-
Hallak M.H, Nomani M.Z.A (1988). Body weight
loss and changes in blood lipid levels in normal men on hypocaloric diets
during Ramadan fasting. Am. J. Clin. Nutr. 48:1197-1210.
Haynes W.G, Morgan D.A, Walsh S.A, Mark A.L, Sivitz W.I
(1997). Receptor-mediated regionalsympathetic nerve activation by
leptin. J Clin Invest; 100: 270-278.
Hoost U, Kelbaek H, Rasmusen H, Court-Payen M,
Christensen NJ, Pedersen-Bjergaard U,Lorenzen T, Haemodynamic (1996).
Effects of eating: the role of meal composition. Clin Sci (Lond); 90:
269-276.
Husain R, Duncan M.T, Cheah S.H, Ch'ng S.L
(1987). Effects of fasting in Ramadan on tropical Asiatic Moslems. Br.
J. Nutr. 58(1):41-48.
Husain R, cheah S.H, Duncan M.T (1996).
Cardiovascular reactivity in malay moslems during Ramadan , SINGAPORE MED J ,
1996, VOL 37 :398-401.
-I-
Ibrahim W.H, Habib H.M, Jarrar A.H, Al Baz S.A
(2008). Effect of Ramadan fasting on markers of oxidative stress and
serum biochemical markers of cellular damage in healthy subjects. Ann. Nutr.
Metab. 53(3-4):175-81.
-J-
JOURDAN G (2008) Consequences of induced and
spontaneous alterations of autonomic nervous system on cardiovascular function
: Physiological and pharmalogical approaches . PhD in pharmacology .
-K-
Kadri N, Amina T, El BatalM, TalitY, Mechakra TahiriS and
MoussaouiD (2000). Irritability During the Month of
Ramadan.Psychosomatic Medicine 62:280-285.
Kamath M.V and Fallen E.L (1993).
Power spectral analysis of heart rate variability: anoninvasive
signature of cardiac autonomic function. Critical reviews in biomedical
engineering.1993, 21, pp. 245-311.
Karli U, Guvenc A, Aslan A, Hazir Tand Acikada C
(2007). Influence of Ramadan fasting on anaerobic performance and
recovery following short time high intensity exercise, Journal of Sports
Science and Medicine 6, 490-497
63
Karpovich P, Sinning W (1980). Physiology of
muscle activity , Vigot Edition Paris.
64
Kearney M.T, Cowley A.J, Stubbs T.A, Perry A.J,
MacDonald I.A. (1996). Central and peripheralhaemodynamic responses to
high carbohydrate and high fat meals in human cardiac transplantrecipients.
Clin Sci (Lond); 90: 473-483.
Kirkendall D.T, Leiper J.B, Bartagi Z, Dvorak J,
Zerguini Y (2008). The influence of Ramadan on physical performance
measures in young Muslim footballers. J. Sports Sci. 26 Suppl 3:S15-27.
-L-
Lam F.Y, Wilson A.T, Channer K.S (1996). The
effect of meals of differing composition on exercisetolerance in patients with
angina pectoris. Eur Heart J; 17: 394-398.
Langford E.J, Ishaque M.A, Fothergil J, Touquet R
(1994). The effect of the fast of Ramadan on accident and emergency
attendances. J R Soc Med 87:517-518
Langley J.N (1921). The Autonomic Nervous
System. Cambridge, Angleterre: Heffer & Sons.
Léger L.A, Lambert J (1982). A maximal
multistage 20-m shuttle run test to predict VO2 max. Eur. J. Appl. Physiol.
Occup. Physiol. 49(1):1-12.
Leiper J.B, Junge A, Maughan R.J, Zerguini Y, Dvorak J
(2008). Alteration of subjective feelings in football players
undertaking their usual training and match schedule during the Ramadan fast. J.
Sports Sci. 26(S3):S55-S69.
Leiper J.B, Molla A.M, Molla A.M (2003). Effects
on health of fluid restriction during fasting in Ramadan. European J Clin Nutr
57(Suppl 2):S30-S38
Low P.A (1997). Clinical autonomic disorders.
Philadelphia: Lippincott-Raven, 1997.
-M-
Malpas S.C (2004). What sets the long-term level
of sympathetic nerve activity: is there a role forarterial baroreceptors? Am J
Physiol Integr Comp Physiol; 286: R1-R12.
65
Malliani A, Pagani M, Lombardi F and Cerutti S
(1991). Cardiovascular neural regulation explored in the frequency
domain. Circulation. 1991, 84 (2), p. 482-492.
MathiasC.J (2000). Neurology in clinical
practice.Boston: Butterworth-Heinemann, 2000. pp. 231-265.
Mathias C.J, Bannister R (2002). Autonomic
failure: a textbook of clinical disorders of the autonomic nervous system.
Oxford: Oxford University Press, 2002.
Matsumura K, Tsuchihashi T, Fujii K, Abe I, Iida M
(2002). Central ghrelin modulates sympatheticactivity in conscious
rabbits. Hypertension; 40 : 694-699.82
Matsumura K, Tsuchihashi T, Fujii K, Iida M
(2003). Neural regulation of blood pressure by leptin andthe related
peptides. Regul Pept; 114: 79-86.
Maughan R.J (2010). Fasting and sport: an
introduction. Br. J. Sports Med. 44 (7) : 473-5.
Maughan R..J, Bartagi Z, Dvorak J, Zerguini Y
(2008a). Dietary intake and body composition of football players
during the holy month of Ramadan. J. Sports Sci. 26 Suppl 3:S29-38.
Maughan R.J, Leiper J.B, Bartagi Z, Zrifi R, Zerguini
Y, Dvorak J (2008b). Effect of Ramadan fasting on some biochemical and
haematological parameters in Tunisian youth soccer players undertaking their
usual training and competition schedule. J. Sports Sci. 26 Suppl 3:S39-46.
McGregor S.J, Hulse M, Strudwick A (2002).
The reliability and validity of two soccer skills tests. In W Spinks, T Reilly,
A Murphy (Eds.), Science and football IV (pp. 300-303). London: Routledge.
Mc Murray G, Proctor C.R , Wilson W.L (1991).
Effects of caloric deficit and dietary Manipulation on aerobic and anaerobic
exercise. Int J Sport ,Med . 12:167-172.
66
Meckel Y, Ismaeel A, Eliakim A (2008). The
effect of the Ramadan fast on physical performance and dietary habits in
adolescent soccer players. Eur. J. Appl. Physiol. 102(6):651-657.
Mizuno K, Asano K, Okamoto K (1998). Effect
of night exercise on the following partially deprived sleep. Psychiatry Clin.
Neurosci. 52 (2) : 137-138.
Montain S.J, Hopper M.K, Coggan A.R, Coyle E.F
(1991). Exercise metabolism at different time intervals after a meal.
J Appl Physiol; 70: 882-888.
Montano N, Ruscone T.G., Porta F.A..Lombardi, Pagani
M., and Malliani A. (1994). Power spectrum analysis of heart rate
variability to assess the changes in sympathovagal balanceduring graded
orthostatic tilt. Circulation. 1994, 90, pp. 1826-1831.
Mougin F, Bourdin H, Simon-Rigaud M.L, Didier J.M,
Toubin G, Kantelip J.P (1996). Effects of a selective sleep
deprivation on subsequent anaerobic performance. Int. J. Sports Med. 17 (2) :
115-9.
Murphy P.J, Campbell S.S (1997). Nighttime
drop in body temperature: a physiological trigger for sleep onset, Sleep 20 (7)
: 505-511.
Neto Souza E.P,Neidecker J , Lehot J.J
(2003). To understand blood pressure and heart rate variability.
Annales Françaises d'Anesthésie et de Reanimation, 22 (2003)
425-452
-N-
Nieman D.C, Carlson K.A, Brandstater M.E, Naegele R.T
(1987). Blankenship JW. Running endurance in 27-h-fasted humans. J
Appl Physiol; 63: 2502-2509.
-P-
Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R,
Pizzinelli P, Sandrone G, Malfatto G, Dell'Orto S, Piccaluga E, et al.
(1986). Power spectral analysis of heart rate and arterial pressure
variabilities as a marker of sympatho-vagal interaction in man and conscious
dog. Circ Res 59: 178-193
67
Pincivero D.M, Lephart S.M, Karunakara R.A
(1997). Reliability and precision of isokinetic strength and muscular
endurance for the quadriceps and hamstrings. Int J sports Med , 18
:113-7Pocock G, Richards C (2004). Autonomic nervous
system.Human physiology - The basics of medicine . Paris: Masson; 2004. p.
175-84.
Pruvost M (2007). Convulsive Seizures and
Autonomic Nervous System Analysis of Cardiorespiratory Coordination by
Spectral, Geometric and Symbolic Methods. PhD in Biological and Medical
Engineering, Specialty: Signal Processing.
-R-
Ramadan J (2002). Does fasting during Ramadan
alter body composition, blood constituents and physical performance .Med.
Princ. Pract. 2:41-46.
Ramadan J, Telahoun G, Al-Zaid N.S, Barac-Nieto M
(1999). Responses to exercise, fluid and energy balance during Ramadan
in sedentary and active males. Nutrition 15 : 735-739.
Reilly T, Waterhouse J (2007). Altered
sleep-wake cycles and food intake: the Ramadan model. Physiol. Behav. 90(2-3) :
219-28.
Reilly T, Waterhouse J (2009). Sports
performance: is there evidence that the body clock plays a role? Eur. J. Appl.
Physiol. 106 (3) : 321-32.
Rimoldi O, Pierini S, Ferrari A, Cerutti S, Pagani M,
and Malliani A (1990). Analysisof short-term oscillations of RR and
arterial pressure in conscious dogs. American Journal ofPhysiology. 1990, 258,
pp. H967-H976.
Roky R, Chapotot F, Benchekroun M.T, Benaji B, Hakkou
F, Elkhalifi H, Buguet A (2003) Daytime sleepiness during Ramadan
intermittent fasting: polysomnographic andquantitative waking EEG study. J.
Sleep Res. 12:95-101.
Roky R, Chapotot F, Hakkou F, Benchekroun MT, Buguet A
(2001). Sleep duringRamadan intermittent fasting. J. Sleep Res.
10:319-327.
68
Roky R, HoutI, Moussamih S, Qotbi S, & AadilN
(2004).Physiological and chronobiological changes during Ramadan
intermittent fasting. Annals of Nutrition and Metabolism, 48, 296-303.
Rösch D, Hodgson R, Peterson T.L, Graf-Baumann T,
Junge A, Chomiak J, Dvorak J (2000) Assessment and evaluation of
football performance. Am. J. Sports Med. 28 (5Suppl):S29-39.
-S-
Schachinger H, Weinbacher M, Kiss A, Ritz R, Langewitz
W (2001). Cardiovascular indices of peripheral and central sympathetic
activation. Psychosom Med; 63: 788-796.
Shanks N.J, Ansari M, & al-Kalai D
(1994). Road traffic accidents in Saudi Arabia. Public Health, 108,
27-34.
Shirreffs S.M, Sawka M.N, Stone M (2006).
Water and electrolyte needs for football training and match-play. J. Sports
Sci. 24 (7) : 699-707.
Siddiqui Q.A, Sabir S, Subhan M.M (2005). The
effect of Ramadan fasting on spirometry in healthy subjects. Respirology
10:525-528.
Smith A, Maben A, Brockman P (1994) Effects
of evening meals and caffeine on cognitive performance, mood and cardiovascular
functioning. Appetite 22(1):57-65.
Sobhani I, Rigaud D, Merrouche M, Vatier J
(1997) The digestive and nutritional changes induced by Ramadan
fasting .Methodologicalrequirements and relevance of scientific observations .
Gastroenterol. Clin. Biol. 21:811-812.
Souissi N, Bessot N, Chamari K, Gauthier A,
Sesboüé B, Davenne D (2007a). Effect of day time on
aerobic contribution to the 30-s Wingate test performance. Chronobiol. Int.
N24(4):739-48.
69
Souissi N, Souissi H, Sahli S, Tabka Z, Dogui M, Ati
J, Davenne D (2007b). Effect of Ramadan on the diurnal variation in
short-term high power output. Chronobiol. Int.24(5):991-1007.
Souissi N, Souissi M, Souissi H, Chamari K, Tabka Z,
Dogui M, Davenne D (2008). Effect day time and partial sleep
deprivation on short-term, high-power output. Chronobiol. Int.
25(6):1062-76.
Spalding J. M. (1969). Handbook of clinical
neurology. Amsterdam:Elsevier, 1969. pp. 107-127. Stannard S.R,
Thompson M.W (2007) the effects of participation in Ramadan on
substrate selection during submaximal cycling exercise. J Sci Med Sport (in
press)
Stokholm K.H, Breum L, Astrup A (1991).
Cardiac contractility, central hemodynamics and blood pressure regulation
during semistarvation. Clin. Physiol. 11:513-523.
Sweileh N, Schnitzler A, Hunter G.R, Davis B
(1992). Body composition and energy metabolism in resting and
exercising Muslims during Ramadan fast. J. Sports Med. Phys. Fitness
32:156-163.
Symons J.d, VanHelder T and Myles W.S (1988).
Physical performance and physiological responses following 60 hours of sleep
deprivation .Med Sci Sports Exerc20:374-80.
-T-
Taoudi Benchekroun M, Roky R, Toufiq J,Benaji B,
Hakkou F (1999). Epidemiological study : chronotype and daytime
sleepiness before and during Ramadan .Therapie 54:567-572.
-V-
Vandewalle H, Peres G, Heller J, Panel J, Monod H
(1987). Force-velocity relationship and maximal power on a cycle
ergometer. Correlation with the height of a vertical jump. Eur. J.Appl.
Physiol. Occup. Physiol. 56(6):650-6.
VanHelder T, Radomski M.W (1989). Sleep
deprivation and the effect on exerciseperformance. Sports Med. 7(4):235-47.
70
-W-
Waterhouse.J (2010). Effects of Ramadan on
physical performance: chronobiological considerations. Br. J. Sports Med. 44
(7): 509-15.
Whitley H.A, Humphreys S.M, Campbell I.T, Keegan M.A,
Jayanetti T.D, Sperry D.A, MacLaren D.P, Reilly T, Frayn K.N (1998).
Metabolic and performance responses during endurance exercise after high-fat
and high-carbohydrate meals. J Appl Physiol ; 85: 418-424.
Wilbert-LampenU, LeistnerD, GrevenS, PohlT, Sper S,
VolkerC, GuthlinD, Plasse A, KnezA, KuchenhoffH, and SteinbeckG
(2008). Cardiovascular Events During World Cup Soccer. N.Engl.J Med.
l-31-;358(5):475-83.
Wilmore J.H, Costill D.L (1998). Physiology of
sport and exercise. University Boeck .
-Y-
Yamamoto Y, Hughson R.L, Peterson J.C (1991).
Autonomic control of heart rateduring exercise studied by heart rate
variability spectral analysis. J Appl Physiol 71: 1136- 1142.
Young J. B. & Landsberg L (1980). Impaired
suppression of sympathetic activity during fasting in the gold
thioglucose-treated mouse. Journal of Clinical Investigation 65, 1086-1094.
Yucel A, Degirmenci B, Acar M, Albayrak R, Haktanir A
(2004). The effect of fasting on Ramadan on the abdominal fat
distribution: assessment by computed tomography. Tohoku J.Exper. Med.
204:179-187.
-Z-
Zerguini Y, Kirkendall D, Junge A, Dvorak J
(2007). Impact of Ramadan on physical performance in professional
soccer players. Br. J. Sports Med. 41:398-400.
71
Ziaee V, Razaei M, Ahmadinejad Z, Shaikh H, Yousefi R,
Yarmohammadi L, Bozorgi F, Behjati M. (2006). The changes of metabolic
profile and weight during Ramadan fasting.Singapore Med. J. 47:409-414.
Zinker B.A, Britz K, Brooks G.A (1990).
Effects of a 36-hour fast on human endurance and substrate utilization.J Appl
Physiol; 69:1849-1855
Zoladz J.A, Konturek S.J, Dudai K, Majercza J,
Sliwowski Z, Grandys M, Bielanski W (2005). Effect of
moderate incremental exercise , performed in fed and fasted state on
cardio-respiratory variables and leptin and ghrelin concentrations in young
healthy men, J. of Physiology and Pharmacology 2005, 56, 1, 63.85.
Abstract
Background
The evaluation of the autonomic nervous system through the
analysis of the heart rate variability has been used by a lot of research teams
during these last two decades but the effects of Ramadan have always been a
matter of controversy, especially, on children and adolescents. And since there
were no anterior studies on this specific subject, our protocol tried to
establish a connection between RF and the autonomic nervous system.
Aim
Recognize the effects of Ramadan on BMI, on anaerobic sports
performance through a laboratory test (the Wingate test) and on the activity of
the autonomic nervous system through the analysis of heart rate variability.
Methods
Nine male athletes playing football for at least five years in
a professional league club, aged 16.2 #177; 0.5 years, weighing 66 #177; 6 kg
and a size of 176 #177; 5 cm, participated in our study. This study took place
during the summer of 2011 during the month of Ramadan where the duration of the
fast varies between 14h30 and 15h. The measurements were done between 3:00 PM
and 5:00 PM. On all sessions these parameters were measured/calculated: Height
(cm), Weight (kg), BMI (Kg/m2), Performances during a Wingate test,
and the HR beat to beat using a Polar S810 device. The HRV was measured during
three maneuvers: supine position, standing position and during the effort. To
analyze the results we transferred the data on computer and we used the
software (The Biomedical Signal Analysis Group, Applied Physics Department,
University of Kuopio, Finland) to process them.
Results
Neither the BMI nor the performances during Wingate test were
influenced by RF. The parameter that was significantly lowered during RF was
the HR at rest and during the effort. In addition, our results showed a
modulation of the vegetative nervous system which leans towards the
parasymapatic tone.
Conclusion:
RF did not bring any significant changes on the anthropometric
parameters and the anaerobic performances of our subjects but influence the
sympathetico-vagal balance.
Keywords
Ramadan fasting - Wingate Test - Autonomic Nervous System - Heart
Rate Variability
| 


