3.1.3 Overall plankton abundance
The study sites exhibited seasonal variation in the abundance
of phytoplankton and zooplankton (Fig 3.7 and Fig. 3.8). The highest abundance
of zooplankton was recorded in February while the highest abundance of
phytoplankton was recorded in April.
A clustering of stations according to plankton abundance using
complete linkage is presented in Fig 3.9 and Fig.3.10. These figures show
Sibasa and Mpopoma reservoirs' abundance being very dissimilar to all other
reservoirs in February while Sibasa, Makoshe and Mezilume are very dissimilar
to others in April. No similarity is found between reservoirs according to
their presence or not in the National Park or in the communal lands. A first
level similarity is found in February between Chitampa (National Park), Makoshe
and Dewa (Communal Lands) similarity in abundance of stations according to
their occurrence in the National Park or in the communal lands is depicted.
However, these Figures show how close the studied reservoirs were in terms of
abundance. Different linkages between the two sampling periods can be found as
well when comparing these clusters.
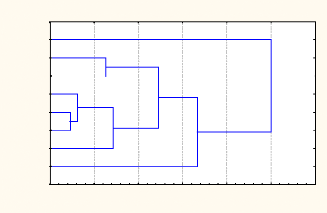
CHITAMPA
MEZILUME
MPOPOMA
MAKOSHE
MALEME
SIBASA
DENJE
DEWA
0 20 40 60 80 100 120
(Dlink/Dmax)*1 00
February
Fig. 3.9 Clustering of stations according to the overall plankton
abundance/ February

MPOPOMA
CHITAMPA
MAKOSHE
MEZILUME
MALEME
SIBASA
DENJE
DEWA
0 20 40 60 80 100 120
(Dlink/Dmax)*1 00
April
Fig. 3.10 Clustering of stations according to the overall
plankton abundance/ April
3.1.4 Relationships between physico-chemical parameters and
plankton species in the studied reservoirs
A comparison of the National Park and communal lands for a
number of parameters and species relationships showed significant differences
(annexes 5 and 6). The following elements showed a significant difference
taking Mezilume (National Park) and Makoshe (Communal lands) as an example: pH
of water (P<0.01), electroconductivity of water (P<0.001), total nitrogen
(P <0.05), the cladoceran Moina (P <0.05), the cyanophyte
Anabaena (P <0.05), and the dinophyte Ceratium (P
<0.05).
The colour of water may have had an influence on the following
elements using ANOVA: pH of water (P<0.01), electroconductivity of water
(P<0.001), total nitrogen (P<0.0 1), hardness of water (P<0.0 1),
soil' s electroconductivity (P<0.0 1), the species Daphnia
(P<0.001), Brachionus (P<0.01), Microdides (P
<0.05), nauplii cyclopoids (P <0.05), nauplii calanoids (P <0.05),
Anabaena (P<0.01), Peridinium (P<0.001),
Melosira (P <0.05), Scenedesmus (P <0.05),
Pediastrum (P<0.01), Hydrodictyon (P<0.01), Dinobryon
(P <0.05), Pleurotaenium (P<0.01), Arthrodesmus (P
<0.05), Fragilaria (P <0.05) and Zygnema (P <0.05).
The soil type also showed a significant influence on some phytoplankton
species.
The clustering of all the components of the main biota in
relation to the water quality is presented on Fig. 3.11 and 3.12. These figures
highlight the relationships of species found in the study area with its
chemistry. Total phosphorus, total nitrogen in the small reservoirs and
electroconductivity of soils had a relationship with zooplankton (Daphnia,
Rotifera, Brachionus and Ostracoda) and phytoplankton taxa
(Navicula, Phacus, Lepadella,
Sphaerocystis, Staurodesmus, Cosmarium and
Sporangium). The pH of water and soils, water hardness and
electroconductivity also influenced some species (Fig. 3.11 and 3.12).

-0.2
-0.4
-0.6
-0.8
0.8
0.6
0.4
0.2
0.0
1.0
-0.6 -0.4 -0.2 0.0 0.2 0.4 0.6 0.8 1.0
TNWATER
ECSOIL ROTIFERA
BRACHION TPWATER
OSTRACOD
TN and TP
DAPHNIA
Factor Loadings, Factor 1 vs. Factor 2
Rotation:
Unrotated
Extraction: Principal axis factoring
CLADOCER
MICRODID
NAUPLCYC
Factor 1
NAUPLCA
CALANOID
KERATLL
CERIODAP MACROTHR
CYCLOPS
PHSOIL
MOINA
HARDNWAT
PHWATER
PH and EC
ECWAT
Fig. 3.11 Relationship between chemistry and zooplankton in the
studied reservoirs
Taxa abbreviations: Nauplii cyclopoids (NAUPLCYC), Nauplii
calanoids (NAUPLCA), Microdides (MICRODID), Cladocera (CLADOCER),
Abiotic elements abbreviations: Total Nitrogen (TNWATER), Total
Phosphorus in water (TPWATER), electroconductivity in soils (ECSOIL), water
hardness (HARDWAT), water' s electroconductivity (ECWAT)
Factor Loadings, Factor 1 vs. Factor 2
Rotation:
Unrotated
Extraction: Principal axis factoring
|
0.6 0.4 0.2 0.0 -0.2 -0.4 -0.6 -0.8 -1.0 -1.2
|
|
|
STAURODE
|
|
|
NAVICULA COSMARIU
|
|
MELOSIRA
|
|
PHACUS TPWATER
|
RHIZOSOL
|
|
ECWAT
|
|
LEPADELL TNWATER
|
|
|
ANABAENA
|
|
|
|
|
HARDNWAT
|
GYROSIGM
|
|
ECSOIL
|
|
PHWATER
|
|
|
|
|
|
SPHAEROC
|
|
|
PH, hardness and EC
|
|
SPORANGI
TN and TP
|
|
|
PHSOIL
|
|
|
|
|
|
CLOSTERI
|
|
|
|
SYNEDRA
|
|
|
|
PERIDINI EUGLENA
|
|
|
|
CERATIUM
|
|
-1.2 -0.8 -0.4 0.0 0.4 0.8
Factor 1
Fig. 3.12 Relationship between chemistry and phytoplankton in the
studied reservoirs.
Taxa abbreviations: Gyrosigma (GYROSIGM),
Lepadella (LEPADELL), Staurodesmus (STAURODE),
Cosmarium (COSMARIO), Rhizosolenia (RHIZOSOL),
Sphaerocystis (SPHAEROC), Sporangium (SPORANGI),
Closterium (CLOSTERI).
Abiotic elements abbreviations: Total Nitrogen (TNWATER), Total
Phosphorus in water (TPWATER), electroconductivity in soils (ECSOIL), water
hardness (HARDWAT), water' s electroconductivity (ECWAT)
| 


